What are the hazards and do they prevent ‘lone working’?
Sulphuric acid: It is a very corrosive chemical that can lead to serious burns when poorly handled especially in its concentrated form. Sulphuric acid produces chemical burns as well as secondary thermal burns attributed to dehydration. Sulphuric acid can corrode the skin, work surfaces and metal. Direct contact with the eyes can cause irreversible blindness, whereas ingestion can lead to the permanent damage of internal organs and death. Making contact with high concentrations of sulphuric acid aerosols can also injure the eye, irritate the respiratory tract and destroy tissues. Long-term exposure to low concentrations of sulphuric acid can erode teeth.
Phosphate buffer: May be hazardous when inhaled, ingested or absorbed by the skin. It can also irritate the eyes, skin or the mucous membranes of the respiratory system.
Sodium acetate buffer: Can pose extreme danger when it touches the skin. It is an irritant that can also affect the eyes and the gastrointestinal tract when ingested. Sodium acetate buffer can also corrode the skin and the mucous membrane when inhaled. A spray mist of the chemical can also damage the mucous membranes found in the eyes, mouth and respiratory system.
These hazards cannot prevent lone working because the level of risk involved is minimal. For example, the sulphuric acid is provided in a ready-to-use dilute concentration, which minimises the risk of injury when preparing dilute acid from the concentrated stock solution. Additionally, the experimental procedures involve pipetting small quantities of the chemicals, which will prevent the formation of aerosols and lower the risk.
What will be done in the procedure?
Each group will prepare working analytical standards from the stock solution whose concentration is 1000ng/mL. Various concentrations will be prepared by diluting a specified quantity of the stock solution in the phosphate buffer. For example, to prepare 100ng/mL, 100µL of the 1000ng/mL stock solution will be mixed with 900µL phosphate buffer at pH 7.2. 50, 10 and 5 ng/mL concentrations will be prepared by taking 500µL of 100ng/mL, 200µL of 50ng/mL and 500µL of 10ng/mL, which will be mixed in 500, 800 and 500µL of phosphate buffer at a pH of 7.2 respectively.
Other concentrations will be prepared as illustrated in the lab manual. Thereafter, subsequent steps will also be done as explained in the manual. The final step will involve the addition of 25 µl of 2.5M sulphuric acid stop solution to each well. The absorbance of the plates will then be measured.
How will the risks be minimised?
The risks will be minimised by wearing protective eye equipment, gloves, as well as protective clothing. Additionally, all activities will be done in a well-ventilated laboratory to reduce the risk of inhaling the toxic chemicals.
Are there any specific risks to females of childbearing age who could become pregnant or any risks to new and expectant mothers?
There are no specific risks to females of childbearing age as well as new and expectant mothers. The reported hazards do not have any carcinogenic or mutagenic effects, which could be dangerous to a developing foetus. In addition, the is no information regarding the excretion of ingested substances through breast milk, which could be dangerous to breastfeeding children.
What is the assessed risk?
The assessed risk is minimal and is mainly attributed to skin contact with hazardous chemicals. This risk will be mitigated by wearing protective gear.
Is health surveillance required?
Health surveillance is not needed because the chemicals and experimental procedure do not pose risks to specific groups of people such as pregnant women or women of childbearing age. Additionally, the risk assessment shows that exposure to the hazardous chemicals is controlled sufficiently.
How will you dispose of the material used?
The materials used will be disposed of based on their physical and chemical nature. For example, non-corrosive liquids will be washed down the sinks with plenty of water, whereas solid waste will be disposed of in a solid waste bin for further incineration in the appropriate incineration facility. The liquid waste will include the reaction mixture in the 96-well plate after the reaction as well as unused dilute reagents. The solid waste will be the plate itself.
Procedure in case of accident or spillage
In case of an accident or spillage, the contaminated surfaces will be rinsed thoroughly with clean water. Protective gear such as gumboots will also be worn when cleaning up the spillage. Absorbent pads will be needed to absorb spills of corrosive substances such as sulphuric acid before further cleaning. A neutraliser such as sodium carbonate in solid or liquid form may be needed. To prevent inhalation of toxic fumes following a spillage, all laboratory occupants will be asked to leave the lab until fresh air circulation is restored. Accidents involving direct skin contact will be mitigated by washing the affected skin with plenty of clean water. In the event of eye contact, the eyes will be flushed thoroughly with clean water before seeking the services of a healthcare provider.
If this procedure will involve ‘lone working’ has the risk assessment taken this into consideration?
The risk assessment has considered the possibility of lone working and ensured that the chemical reagents, particularly the stock solutions, are in moderate concentrations that will not pose a serious risk. Additionally, the experimental procedures minimise the formation of aerosols that could be inhaled or reach the eyes. However, the experiment involves working in groups. Therefore, additional mitigation strategies may not be needed because ‘lone working’ will not take place.
Update: The provided risk assessment is up-to-date. There are no significant changes in the procedures.
Pipette Performance Check
What is the importance of this task?
Checking the performance of a pipette is important to ensure that pipettes are performing within the required specifications to guarantee accuracy and consistency in analytical techniques. Additionally, this process also ensures accurate liquid delivery thereby ensuring that high quality results are obtained at all times. Most laboratory analyses, including immunological techniques, are affected by the concentrations of various reagents that are mixed during analysis. The ratio of antigens to antibodies may affect the extent of binding and the results. Checking the performance of pipettes reduces the risk of faulty results and saves time that could otherwise be wasted on investigating what could have gone wrong in the experiment.
What is GLP?
GLP stands for good laboratory practice. It is a group of rules meant to guarantee the quality and reliability of non-clinical laboratory investigations whose purpose is to back investigative or selling permits for commodities under the regulation of government organisations. GLP relates to the handling of pharmaceutical and non-pharmaceutical substances, including food colours and additives, presence and extent of contamination, food packaging and medical instruments. GLP only applies to non-clinical investigations as well as testing and is a quality management system as opposed to a scientific management system. Therefore, GLP does not describe scientific benchmarks. Crucial aspects captured in GLP include benchmarks for study conduct, the collection of data and recounting of outcomes.
What % error is acceptable when pipetting and is the acceptable % error similar for all pipettes?
The acceptable % error when pipetting is 2% of the full scale at all volume settings. These standards are also followed by the ISO 8655-2 standard during pipette testing. For instance, a 100 μL variable-volume pipette has a systematic error of ±2.0 μL, which is 2% of the total volume at the 100 μL setting. However, when a similar systematic error is assumed at the 50 μL setting, the ±2.0 μL error translates to 4%. This percentage is the same for medium and large volume pipettes. However, the systematic error increases for smaller volumes. This deviation is beneficial in gravimetric techniques to cater for the increased error characteristic of gravimetry at small volumes.
Please define %CV
CV stands for coefficient of variation. It is a proportion of the standard deviation to the mean, expressed out of 100%. CV makes it possible to contrast the extent of variation from one data set to another even in cases where the means differ substantially. The %CV is computed by dividing the standard deviation by the anticipated value and multiplying the quotient by 100. High values of %CV are indications of large variations within a data set, whereas low %CV values show minimal discrepancies. In most experimental investigations, data consistency is desired to ensure the reproducibility of the findings. Therefore, high %CV values are indications that there is a need to troubleshoot the experiment to identify the source of variation.
Define accuracy and precision?
Accuracy denotes how close a measured value is to a specified benchmark or known value. Precision relates to the closeness of two or more measurements to each other. If a packet of flour is weighed ten times and found to be 1.5 kg each time, then the measurement is said to be precise. There is no relationship between accuracy and precision because a wrong or inaccurate measurement can be precise.
For the results generated, the pipette could be considered accurate because the obtained measurements were close to the expected ones based on the acceptable error for pipettes. The errors were within the 2% limit. However, the results were not precise because different values were obtained when the measurements were repeated.
Reporting of ELISA Results
Table of results showing the average response, standard deviation and % CV at each concentration
Table 1. Raw data.
Table 2. Average response, standard deviation (SD) and % CV at each concentration.
A similar table to generate a normalised calibration curve
A calibration curve of normalized response against concentration
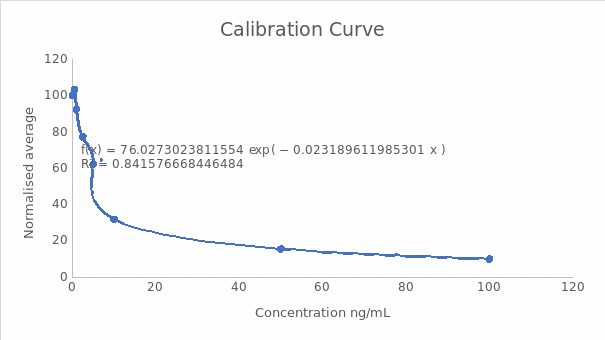
Midpoint (IC50) and the dynamic range (IC20-IC80)
IC50 = ((Normalised response zero standard) – (normalised response highest standard) x0.5) + normalised response highest standard
IC50 = (100-9.91) x 0.5+9.91
ICS50 = 54.96, which is equal to 5.69 ng/mL this parameter indicates the midpoint of the curve and is crucial in determining the sensitivity and specificity of the test.
IC20 = ((Normalised response zero standard) – (normalised response highest
standard) x 0.8) + normalised response highest standard
IC20 =100-9.91 x 0.8+9.91
IC20= 81.98, which is equal to 2.29 ng/mL
IC80 = ((Normalised response zero standard) – (normalised response highest standard) x0.2) + normalised response highest standard
IC80 =100-9.91 x 0.2+9.91
IC80= 27.93, which is equal to 11.36 ng/mL
IC20 to IC80 corresponds to the dynamic range, which is equivalent to normalised responses of 81.98 to 27.93 and concentrations of 2.29 ng/mL and 11.36 ng/mL.
The concentration of chloramphenicol from three unknown samples
From the interpolation of the standard curve, the concentrations of the 3 unknown solutions are as follows:
Unknown 1= 37.75 ng/mL
Unknown 2= 317.34 ng/mL
Unknown 3= 5.078 ng/mL
Comparing results within the group between person 1 and person 2
For person 2, the values of the unknown solutions were as follows:
Unknown 1= 71.73 ng/mL
Unknown 2= 70.03 ng/mL
Unknown 3= 2.51 ng/mL.
These values differed significantly from the main results (person 1). These differences could be attributed to the likelihood of varying concentrations of chloramphenicol in the samples that were tested. Different concentrations of chloramphenicol elicit varying signals as recorded by the range of optical density (OD) values.
Description of the microarray
The microarray is meant for the diagnosis of chloramphenicol in the samples. In A, the first set of two dots shows fluorescence from labelled bovine serum albumin (BSA), which acts as the positive control. A similar set of dots is found at the end of the microarray. The printed buffer spots play the role of negative controls and help to check for any abnormal non-specific binding. Inadequate fluorescence signal on the positive control spots generates an invalid test result.
The BSA control spots have been created to yield fluorescence signal similar to a characteristic positive sample. The second, third, fourth and fifth sets of four dots indicate the fluoresce signal from the analyte-specific antigens, which in this case is 100ug/ml of chloramphenicol conjugate. The extent of fluorescence is comparable to that of the positive controls (BSA signals), which confirms the validity of the test.
In microarray B, the first set of two dots at the beginning and the end of the cartridge print area shows fluorescence from labelled BSA, which is the positive control. The second, third, fourth and fifth sets of four dots indicate very faint fluorescence signal from the analyte-specific antigens, which is also indicated as 100ug/ml of chloramphenicol conjugate. The extent of fluorescence from this set of microarrays is less than that of the positive controls (BSA signals), which shows that the samples in the test area are either negative for chloramphenicol (test analyte) or have very small quantities of the analyte.
5 items to consider before implementing a new immunological method as a screening test for chloramphenicol
Sensitivity: The new immunological method should be sensitive enough to the test to avoid the generation of false positive and negative results.
The limits of detection of the test should be ascertained to ensure that the test can generate results that comply with various food and safety standards. Known limits of detection will enable the effective identification of potential noncompliant samples.
The new immunological method should be capable of high-throughput: It should be possible to process numerous samples within a reasonable timeframe using the new technique.
Cost: The cost-effectiveness of the method should be determined. Several pertinent factors should be considered in the cost. For example, the cost of the test kit and reagents, the cost of interpreting the results, as well as data processing and management costs. Overall, the method should be affordable.
Quality: The test should provide consistent results always to enhance reproducibility. Discrepancies in the quality of the output may have a negative effect on the benefits of the test. For example, poor quality results may lead to the rejection of safe food samples with alleged chloramphenicol contamination or certification of contaminated food samples.