Background Information
Carbon dioxide (CO2) is a colorless, odorless gas that is produced in large quantities during the chemical combustion of fuels. It has been categorized as a greenhouse gas that poses a significant danger to the environment. The continued production of carbon dioxide is inevitable due to the energy demand in many domestic and industrial processes. However, the harmful effects of the gas can be reduced by using the emitted carbon dioxide for other beneficial uses.
One strategy entails the direct capture and sequestration of the gas through nanoporous adsorbent substances or amine-based liquid sorbents (Darunte, Walton, Sholl, & Jones, 2016). Indeed, the chemical transformation of carbon dioxide has gained momentum as an efficient strategy sequestering CO2 (Kim, Kim, Jang, Seo, & Ahn, 2013). Chemical studies show that carbon dioxide is a viable 1-carbon building block that can be transformed into useful substances such as cyclic carbonates, dimethyl carbonates, urethanes, N,N-disubstituted ureas, and formic acid among others.
The production of cyclic carbonates entails combining CO2 and styrene oxide to generate five-membered cyclic carbonates (Yang et al., 2014). The resultant cyclic carbonate has immense uses as electrolytes, polar aprotic solvents, and precursors in numerous chemical reactions. Nonetheless, carbon dioxide is an inert gas, which complicates the fixation process (Kim et al., 2013).
A number of homogeneous catalysts, including liquid catalysts, quaternary ammonium salts, transition metal complexes, and ionic liquid catalysts have been investigated for their catalytic potential in the cyclic addition of CO2 and styrene oxide. The main problem with the homogeneous catalysts is difficulties in separation of the product and recovery of the catalyst. Consequently, heterogeneous catalysts have been proposed for these cyclic addition reactions. However, a significant number of these catalysts demonstrate low activities under harsh conditions, for example, high temperatures, and elevated CO2 pressure.
In addition, some catalysts can only work in the presence of organic solvents or co-catalysts. Therefore, there is a need for an environmentally-friendly heterogeneous catalyst that exhibits high catalytic power under a wide range of conditions, especially in mild conditions. The purpose of this review is to examine different metal-organic frameworks and determine their potential effectiveness for the synthesis of organic or cyclic carbonates from carbon dioxide.
Metal-Organic Frameworks (MOFs)
Metal-organic frameworks (MOFs) are created by the simultaneous polymerization of organic molecules with metal ions or groups of metal ions (Kitagawa, 2014). MOFs have gained popularity as a new type of adsorbent crystalline materials with outstanding zeolite-resembling attributes, for example, easily reached pore volumes, homogeneous microspores, high adsorption capabilities, and considerable internal surface areas (Eddaoudi, Sava, Eubank, Adil, & Guillerm, 2015).
These properties make them suitable contenders for functions such as separation and stowage of gases, magnetism, and as catalysts. MOFs have a modular nature and display facile tunability, which makes them the perfect heterogeneous catalysts with consistent active sites that are formed by their building blocks (Guillerm et al., 2014).
During catalysis, MOFs permit structural similarity that is comparable to that of homogeneous catalysts. Additionally, their elevated surface areas and increased pore volume permit an acceptable extent of structural fine-tuning such as that observed with molecular homogeneous catalysts. On the other hand, their pore volume, ultrahigh surface area, and varied nature all speed up good catalytic activity and precipitous purification (Beyzavi et al., 2014).
The use of MOFs has been demonstrated in different reactions, including Friedel–Crafts type reaction, the Knoevenagel condensation reaction, cyanosilylation of carbonyl compounds and imines, acetalization, oxidation of sulfides, ZnEt2 additions to aldehydes, transesterification, and isomerization (Liu et al., 2014). Lead (Pb) held on MOFs has shown its effectiveness as a catalyst in hydrogenation reactions (Song et al., 2009). Other examples of catalysts that have been studied in carbon dioxide fixation include MOF-5, Mg-MOF-74, and CoMOF-74. Nevertheless, these MOF catalysts have proved to be responsive to air, water, and different temperature conditions, thus restricting their further use. Therefore, investigations on different MOF catalysts to identify resilient and effective ones are still ongoing.
Cyclic Carbonates
Cyclic carbonates serve as green solvents in chemical reactions, precursors in the synthesis of polycarbonates, and electrolytes for lithium-ion batteries (Zhu, Srinivas, Bhogeswararao, Ratnasamy, & Carreon, 2013). At present, extremely noxious phosgene is used in the manufacture of cyclic carbonates. A safer alternative for the manufacture of cyclic carbonates is taking advantage of an insertion reaction to incorporate a CO2 molecule into an epoxide.
Conventional solid catalysts such as mesoporous oxides and zeolites have been used extensively in this regard. Catalysts based on mesoporous SBA-15 have been found to be the most effective in the transformation of CO2 and styrene oxide into cyclic carbonates. Cycloaddition reactions have also used catalysts based on zinc metal complexes, organoantimony halides, porphyrins, aluminum metal complexes, phthalocyanines, dialkyltin methoxide, Mg/Al oxide-based catalysts, and Schiff bases (Zhu et al., 2013).
The reaction of CO2 with epoxides to yield cyclic carbonates and similar substances is reported to be catalyzed by Lewis acid sites (Beyzavi et al., 2014). Based on this observation, it is assumed that solids possessing Lewis acid spots on their surfaces in addition to elevated adsorption capacity for carbon dioxide will demonstrate high catalytic activity for cycloaddition reactions. A number of homogeneous catalysts have been used in the catalytic production of cyclic carbonates.
The major shortcomings with these systems are difficulties in the separation of the catalyst and product, as well as the recycling of the catalysts. To circumvent these restrictions, research efforts have focused on developing heterogeneous catalysts such as metal oxides, zeolites, and functional polymers (Lu, Yang, Liu, & Ma, 2017). Improvements to these catalytic systems depend on the in-depth understanding of the basics of cycloaddition reactions.
Cycloaddition of CO2 to Epoxide Leading to Cyclic Organic Carbonates
The addition of CO2 to cycloaddition reactions is a perfectly atom-efficient reaction. Therefore, it is one of the most effective ways of simulated fixation of carbon dioxide gas. Additionally, this process is beneficial as opposed to the conventional syntheses that use highly noxious and corrosive phosgene. These advantages have stimulated interest in the study of the cycloaddition of CO2 to epoxides. The mechanism for this reaction requires an acid catalyst (which could be a proton or a metal ion) that matches up the epoxide, thus initiating it for nucleophilic attack by the co-catalyst to produce a halo-alkoxide. In most cases, the co-catalyst is a tetraalkylammonium halide.
The halo-alkoxide intermediary is capable of reacting with CO2 via cycloaddition to produce a cyclic carbonate (Beyzavi et al., 2015). The tetraalkylammonium halide co-catalyst is regenerated in the process. The rate law for this reaction has been determined as shown below. Altering the amounts of CO2, catalyst, and co-catalyst produces the rate equation, which shows that the concentrations of the epoxide, carbon dioxide, and catalyst are responsible for a first-order reaction, whereas the co-catalyst concentration is responsible for a second-order reaction.
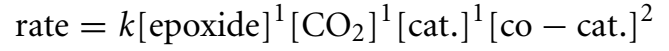
Zeolitic Imidazolate Frameworks (ZIF)
Zeolitic imidazolate frameworks (ZIF) are a subcategory of MOFs with outstanding chemical and thermal stability, which make them potentially good catalyst candidates (Yang et al., 2014). The ZIF-8 framework is characterized by large pore sizes, with an average diameter of 11.6 Å. These pores are reachable through openings of 3.4 Å. ZIF-8 is an attractive compound to use as a catalyst in the conversion of carbon dioxide to cyclic carbonates because of the availability of Lewis acid regions in its structure, particularly zinc ions.
It also has a high adsorption capacity for carbon dioxide, which is attributed to the availability of fundamental sites related to the nitrogen in the imidazole functional group. ZIF-8 demonstrates dynamic catalytic activity in numerous reactions, for example, the transesterification of vegetable oil, the Friedel-Crafts acylation, the Knoevenagel reaction, and the dehydrogenation of dimethylamine borane (Zhu et al., 2013).
The catalytic functioning of ZIF-8 in the manufacture of styrene carbonate using CO2 and styrene oxide was investigated by Zhu et al. (2013). The ZIF-8 system showed substantial catalytic activity at temperatures of approximately 50 °C, which was significantly lower compared to temperatures used in previous experiments using different catalysts. Furthermore, the catalyst could be recycled and used again without significant losses in catalytic potential.
At higher temperatures of 100oC, styrene carbonate yields were approximately 54%, which was comparatively higher than other documented catalysts. Another unique feature of this catalytic reaction was that solvents and co-catalysts were not pre-requisites. The nature and density of the catalyst’s pores in the fresh and reused forms were probed using pyridine and ammonia. Spectroscopic analyses of the adsorbed pyridine showed the availability of Brönsted (B) and Lewis (L) acid sites (Zhu et al., 2013).
However, very few B-sites were available in the reused ZIF-8 catalysts. The adsorption of carbon dioxide on the solid phase and subsequent transformation to cyclic carbonates was enhanced by the simultaneous availability of the acid sites and nitrogen fundamental groups from the imidazole linker in ZIF-8.
These findings corroborated those reported by Miralda, Macias, Zhu, Ratnasamy, and Carreon (2011), who investigated the action of ZIF-8 in the cyclic addition of carbon dioxide and epichlorohydrin. The changeover of epichlorohydrin was 65.5%, whereas the selectivity for the chloropropene carbonate was 63.4%. The output of styrene carbonate was only 53% at temperatures of 100oC. Furthermore, the unique crystalline structure and catalytic activity of ZIF-8 was lost following recycling. Thus, these observations concluded that ZIF-8 could not overcome the stability setback in the production of cyclic carbonates. As a result, there was a need to look for better alternatives.
In a separate study, Yang et al. (2014) evaluated ZIF-68 as a possible heterogeneous catalyst for the chemical fixation CO2. The experimental conditions involved mild conditions of 120oC and 1.00 MPa without any solvents or co-catalysts. Powder X-ray diffraction, N2 adsorption-desorption, and thermal assessments were all used to determine the textural features of the ZIF-68 catalyst. There was a 93.4% yield of cyclic carbonate at the end of 12 hours of the mild reaction state (Yang et al., 2014).
The X-ray diffraction patterns of the recycled catalysts did not reveal any substantial alterations in the crystalline organization when likened to the new catalyst. These findings indicated good stability of ZIF-68 in chemical reactions involving cyclic additions. A I type adsorption-desorption isotherm devoid of hysteresis loop was produced, which insinuated the microporous make-up of the ZIF-68 catalyst. Furthermore, the ZIF-68 catalyst could be reclaimed in three catalytic cycles without any major decrease in catalytic activity.
MOF-5 [Zn4O(BDC)3
On a quest to find MOFs that are effective under mild conditions, Song et al. (2009) performed various reactions using MOF-5 [Zn4O(BDC)3 (BDC is benzene-1,4-dicarboxylate) as the heterogeneous catalyst with quaternary ammonium salts (Me4NBr, Me4NCl, n-Bu4NBr, Et4NBr, n-Pr4NBr). The joint reactions could be done efficiently at mild temperatures and low CO2 pressure. Notable attributes of the catalyst included high activity and selectiveness, affordability, and stability.
Furthermore, it was possible to separate the catalyst and recycle it. However, the activity of MOF-5 was influenced by the presence and type of ammonium salts. Thus, the failure to include an ammonium salt in the reaction mixture led to no detection of products. On the other hand, quaternary ammonium salts activated the catalyst leading to high propylene carbonate yields. The increase was directly proportional to the alkyl chain length of the ammonium salt increased.
Thus, the highest yields were obtained with n-Bu4NBr. This observation was attributed to the configurations of the quaternary ammonium ions, which perhaps affected the performance of the anions. An outstanding synergistic effect in enhancing the reaction was noted between MOF-5 and quaternary ammonium salts. The best yields were obtained at temperatures of approximately 50oC. High levels of selectivity in the reactions were noted at low carbon dioxide pressures within 6 hours.
Additionally, there was no significant loss of activity even after using the catalyst three times, which suggested that the catalyst was very stable. The MOF-5/n-Bu4NBr catalytic system also demonstrated high levels of activity and selectivity for the cycloaddition of carbon dioxides with other epoxides, including epichlorohydrin, glycidyl phenyl ether, and styrene oxide (Song et al., 2009).
Hafnium-Based Metal-Organic Framework
NU-1000 is a novel Zr-based MOF that is found by the solvothermal reaction between “ZrCl4 and 1,3,6,8- tetrakis (p-benzoic acid) pyrene (H4TBAPy) with benzoic acid as a modulator” (Beyzavi et al., 2014, p. 15861). This MOF has unique features such as a high density of acidic zones, constant perviousness with ultra-large channels, a large surface area, and unusual chemical stability, which implied that it could serve as an exceptional catalyst system that can surmount the challenges observed in the formation of cyclic carbonates from carbon dioxide.
By contrasting the dissociation energies of ordinary Hf-O against Zr-O bonds (802 as opposed to 776 KJ Mol-1), Beyzavi et al. (2014) conjectured that Hf had stronger M-O bonds (i.e. was more oxophilic) than Zr and was expected to work as a stronger Brønsted acid. Therefore, it was postulated that a Hf-cluster-based NU-1000 would show higher levels of efficiency in catalysis in the acid-catalyzed schemes compared to the Zr-cluster-based NU-1000. This supposition was evaluated by making a Hf-based MOF (Hf-NU-1000) that maintained the typology of NU-1000. The system was optimized through various simulations.
The Hf-NU-1000 system displayed highly efficient catalytic action for the quantitative cycloaddition of styrene oxide using carbon dioxide at 1a pressure of 1 atmosphere, leading to the formation of styrene carbonate at room temperature. This reaction was the mildest and most efficient catalytic system ever reported for this chemical reaction. The pure product could be achieved following an uncomplicated aqueous extraction because the conversion of epoxide to carbonate a complete and quantitative step.
This simple separation eliminates the need for labor-intensive purification procedures, for example, distillation, which can lead to the disintegration of the product and formation of unwanted byproducts. It was also noted that the efficiency of NU-1000 was lower than that of Hf-NU-1000 under similar reaction conditions, which confirmed the supposition about the comparative catalytic actions of the two systems.
The Hf-NU-1000 system showed superior performance compared to other MOFs, including MOF-5, ZIF-8, ZIF-68, F-IRMOF-3, MIL68(In)-NH2, CoMOF-74, and Mg-MOF-74 (Beyzavi et al., 2014). In addition, the catalyst was recycled five consecutive times without a notable reduction in the efficiency of the catalyst or structural disintegration as demonstrated by the X-ray diffraction analysis. Hf-NU-1000 also demonstrated highly regioselective azidolysis of epoxides as well as a high yield and full conversion of substrate, which had not been reported before.
Nbo Metal-Organic Framework (MOF) Platform MOF-505
Even though a number of existing MOFs have already been tested as heterogeneous Lewis acid catalysts for the chemical transformation of carbon dioxide, conditions such as high temperature and pressure are needed to attain high-efficiency rates (Gao et al., 2014). These preconditions can be related to low densities of active sites in those MOFs. A high density of catalytically active sites can be achieved in MOFs by decorating apices, borders, and faces of polyhedral enclosures in MOFs with catalytically active centers, thus creating MOF-based nanoreactors.
Such nanoreactors demonstrate a high density of active sites in the enclosed nanospace and permit the active sites to angle well in the direction of cage center, which enhances the associations between the substrate and the active sites. Catalytic and coupling reactions of epoxides and carbon dioxide would then be expected under mild conditions.
Gao et al. (2014) aimed at developing a MOF-based nanoreactor that could transform carbon dioxide into cyclic carbonates efficiently at room temperature under a pressure of 1 atmosphere. The proposed technology to attain this objective was through crystal manipulation of the nbo MOF platform with a custom-created azamacrocycle ligand. This alteration led to a high density of well-arranged Lewis active regions in the cuboctahedral enclosure in MMCF-2, [Cu2(Cu-tactmb)(H2O)3(NO3)2].
The resultant MOF showed high catalytic performance in the chemical fixation of carbon dioxide into cyclic carbonates at approximately 25oC at a pressure of less than 1 atmosphere. This performance exceeded that of the parent MOF-505 as well as other copper-based MOFs of HKUST-1 (Gao et al., 2014). It was expected that the crystal engineering strategy for the creation and orientation of a high concentration of catalytically active regions in the constricted nanospace through the customized drafting of functional ligands would be a widely accepted way for the creation of novel classes of highly competent heterogeneous catalytic systems to fix carbon dioxide and other similar reactions.
Ultra-stable Porphyrin Zr Metal-Organic Frameworks
Metalloporphyrins show outstanding catalytic activities. Thus, numerous studies have been done regarding the embedding of porphyrin catalysts in polymers and zeolites (Feng et al., 2013). MOFs deliver an extraordinary platform for the efficient use of porphyrinic catalytic centers because of their homogeneous but adjustable pore sizes. In recent times, porphyrin byproducts have been inserted into MOFs through encapsulation or linker augmentation.
Investigations into the catalytic and optical attributes of these porphyrinic MOFs show that they have comparatively weaker chemical stabilities compared to other MOF catalysts. The chemical instability is linked to their construction process, which uses mild acids and harsh bases. As a result, the use of porphyrinic MOFs in catalysis can only be beneficial in mild reaction conditions. A chemically stable MOF is mandatory for the investigation of their catalytic properties.
Feng et al. (2013) blended the adaptability of metalloporphyrins and the steadiness of Zr-carboxylate MOFs to develop an iron porphyrin zirconium MOF known as PCN-224(Fe). The abbreviation PCN means porous coordination network. A previously developed PCN-222(Fe) demonstrated high levels of stability and peroxidase-resembling catalytic actions. Feng et al. (2013) took advantage of the previous framework to develop a PCN-224 series consisting of four systems that used either nickel, cobalt, iron, or no metal.
The PCN-224 series exhibited the greatest Brunauer-Emmett-Teller surface area of 2600 m2/g in all documented porphyrinic MOFs. Additionally, it was stable in a pH range of 0 to 11. PCN-224(Co) showed great catalytic activity for the coupling reaction between carbon dioxide and propylene oxide, thus showing that it could be applied as a recoverable heterogeneous catalyst.
PCN-224(M) showed appreciable levels of chemical and thermal stability due to the presence of three-dimensional open channels made from multifunctional porphyrin groups. Therefore, it met most of the preconditions for the perfect platform for heterogeneous catalysis. PCN-224 permitted the expedient incorporation of different metals for various types of catalysis and other uses under chemically unfriendly states due to the presence of a free porphyrin as the building block. PCN-224(M) is a fabrication of metalloporphyrins, which gives uniformly dispersed catalytic cores that differ from metalloporphyrins held in porous materials.
Additionally, as the three-dimensional nanochannels facilitate rapid diffusion and carriage of substrates and products, it is expected that the catalytic efficiency will be better than MOFs with smaller pores. Finally, the outstanding stability over a wide range of pH facilitates the use of PCN-224(M) as a recyclable heterogeneous catalyst under severe reaction states. It is also possible to isolate the catalyst from the reaction mix, which contributes towards catalytic efficiency because disintegration of the catalyst and associated contaminations are avoided. During the development of porphyrin-based MOFs, metals could be added to the porphyrin macrocycles of the linkers.
The same metals used in the secondary building unit (SBU) could be used in this process. Such an approach could be used to develop a three-dimensional nanospace with a high density of metal zones, which would enhance catalysis if both metal centers displayed catalytic activity.
A separate study by Gao, Wojtas, and Ma (2014) examined a porous metal-metalloporphyrin framework (MMPF-9) for the fixation of carbon dioxide in the production of cyclic carbonates. MMPF-9 has a high density of Cu(II) regions in the restricted nanospace, which proves that it is a very effective Lewis-acid heterogeneous catalyst suitable for the chemical fixation of carbon dioxide at room temperature and pressures lower than 1 atmosphere.
Gao et al. (2014) studied the functioning of MMPF-9 in the chemical fixation of carbon dioxide with various epoxides whose functional groups had been changed under the appropriate conditions. A high catalytic action was observed for the cycloaddition of butylene oxide and carbon dioxide to produce butylene carbonate at room temperature and a pressure of 1 atmosphere. A yield of 80.3% was obtained by the end of 48 hours.
It was also noted the yield of the resultant carbonate was indirectly proportional to the molecular sizes of the epoxide substrate. This observation was linked to the constricted diffusion of large epoxide molecules, which indicated that MMPF-9 demonstrated size-selective catalysis. However, the output of cyclic carbonate rose from 30.5% to 65.9% when butyl glycidyl ether was used as the substrate following the extension of the catalytic duration from 48 hours to 96 hours (Gao et al., 2014). MMPF-9 could also be reused without a substantial decline in the catalytic activity.
Triazole-Containing MOFs
Li et al. (2016) produced a highly porous MOF n, which included unsaturated Cu regions as well as easily reached nitrogen-rich triazole entities with a high affinity for carbon dioxide. The catalyst exhibited very high efficiency on the catalytic cycloaddition of carbon dioxide with small epoxides at a pressure of 1 atmosphere and room temperature. The assembled MOF containing carbon dioxide-adsorbing attributes and uncovered Lewis-acid metal regions could act as a perfect catalyst for reactions involving carbon dioxide fixation.
However, using larger substrates led to a sharp decline in the catalytic activity, which showed that CO2 cycloaddition of small substrates by the MOF catalyst was performed within the framework, whereas large substrates could not access the porous framework for catalytic activity. Therefore, the synthesized MOF showed high catalytic discrimination to various substrates based on the restriction of the pore width.
Polyoxometalate-Based Homochiral MOFs
Given that light olefins are the cheapest and most readily available chemical building block for the synthesis of cyclic carbonates with carbon dioxide, a one-vessel reaction that deals with an olefin through a sequence of distinct catalytic stages with numerous changes using one diagnosis stage is highly desired for the environmentally friendly transformation of carbon dioxide to useful chemicals (Han et al., 2015). Inherent enzymatic reactions have motivated scientists to mimic natural reactions by formulating automatic catalytic reactions where catalysis facilitates the conveyance of the substrate through mechanistically discrete reactions without having to add more reagents and catalysts or change the reaction state.
Recent investigations have led to the discovery of homogeneous auto-tandem catalysts based on organic and organometallic frameworks, which demonstrate high levels of efficacy in solution (Liu, Xi, Sun, Yang, & Gao, 2016).
This trait can be beneficial in the separation and recovery of the catalyst from the reaction mix by developing heterogenized catalysts with the aid of linkers to connect them to a prop. An example of a solid prop is a metal oxide material with a large surface area. Apart from immobilization on a support, the catalysts can be incorporated in porous MOFs. Thus, the main problem for the creation of auto-tandem catalytic processes has been ensuring that the reaction intermediates are compatible and achieving synergy of the various catalytic cycles.
Polyoxometalates (POMs) are renowned catalysts that have been examined in olefin epoxidation. They have outstanding thermal and oxidative stability in the face of oxygen donors. Blending chiral organic catalysts and POM constituents into one MOF (POMOF) can trigger the lopsided dihydroxylation of aryl alkenes with exceptional stereoselectivity. Han et al. (2015) believed that blending Lewis acid catalysts, chiral organic catalysts, and oxidation catalysts could be a forceful strategy to create a sequential catalytic procedure for the chemical conversion of alkenes to chiral cyclic carbonates.
The single POMOF created by Han et al. (2015) consisted of [ZnW12O40]6- anions, NH2-bipyridine (NH2-BPY), zinc(II) ions, NH2-functionalized bridging links, and pyrrolidine-2-yl-imidazole (PYI), which led to the development of two novel enantiomorphs of POMOFs (ZnW PYI1 and ZnW PYI2). Preliminary tests of the catalyst showed a satisfactory yield of 92% and enantioselectivity as “79% enantiomer excess (ee)) for (R)-styrene oxide” (Han et al., 2015, p.3).
It was not possible for L-PYI together with its hydrochloride salt to trigger the reaction in the same reaction conditions. Using L-PYI and Zn3[ZnW12O40] as catalysts led to conversion rates of 55% and an enantiomer excess 18%. The higher conversion rate in the first instance was linked to the appropriate distribution of sets of the chiral PYI group and the ZnW12O406- oxidant catalyst.
The mechanical minimalism of the building units and the usage of cheap and accessible chemical substances make this method viable for the formulation of practical homochiral catalysts for the conversion of carbon dioxide. However, there is inadequate information regarding the efficacy of this catalytic system, as well as the ability to withstand varying reaction conditions. Future studies are needed to ascertain these variables.
Acylamide-Containing MOFs
Hypothetical and investigational studies have shown that exposed nitrogen-rich functional groups in compounds such as imidazole, amine, tetrazole, triazole, as well as pyridine groups in porous substances, could lead to a substantial increase in the carbon dioxide adsorption capacity (Hug, Stegbauer, Oh, Hirscher, & Lotsch, 2015).
Based on the fact that acylamide as a characteristic nitrogen-rich moiety exhibits a high affinity for carbon dioxide, it was expected that acylamide-containing MOFs with exposed metal zones should have a high potential for the chemical conversion of CO2. Li et al. (2017) developed a MOF containing open nitrogen-rich moieties and free metal sites via the solvothermal construction of an acylamide-containing tetracarboxylate ligand and Cu(II) ions n.
The characterization of the resulting MOF showed that it was highly porous with accessible Lewis acid metal sites as well as a high CO2-adsorbing ability. These features are known to enhance MOFs’ efficiency as a heterogeneous catalyst for the chemical conversion of carbon dioxide. The experimental outcomes showed that the catalyst was highly efficient on the CO2 cycloaddition with epoxides of small molecular sizes.
The open porous windows were able to regulate the size of molecules that could access them. Thus, there was a big difference between the catalytic activity of small and large molecules. Propylene carbonate yields were highest with this catalyst. The evaluation of the catalyst’s recyclability showed that there was no significant reduction in the catalytic action, even after 5 cycles of reactions (Li et al., 2017).
The X-ray diffraction patterns of the reused catalyst were consistent with the computed ones from the original crystal data, which showed that the catalytic framework of the MOF was upheld throughout the reactions. The unusually high efficacy and size discrimination of the MOF on the catalytic conversion of carbon dioxide make it possible for the MOF to be applied as a cutting-edge heterogeneous catalyst for carbon fixation.
Polyoxovanadate-Resorcin[4]arene-Based Porous MOFs
Polyoxometalate (POM)-based MOFs have been identified as having the potential to be good heterogeneous catalysts. This group of catalysts is usually abbreviated as PMOFs. Lu et al. (2017) developed a PMOF [Co2L0.5V4O12]·3DMF·5H2O by using a resorcin[4]arene ligand, which has wheel-like features. The resultant catalyst contained a well-designed porous pattern and was a unique example of PMOFs consisting of a resorcin[4]arene ligand in addition to polyoxovanadate. The PMOF had exposed V sites in the channel, which enhanced its strength as a heterogeneous Lewis acid catalyst by increasing its conversion efficiency and selectivity in reactions involving the cycloaddition of carbon dioxide to epoxides.
The catalytic functioning of the Lewis acid catalyst 1 was assessed for the cycloaddition of CO2 with epoxides at pressures of 1 and 20 atmospheres. It was noted that four substrates 1,4-butanediol diglycidyl ether, benzyl glycidyl ether, glycidyl phenyl ether, and 2-(butoxymethyl) oxirane could be converted in full into the matching cyclic carbonates in 6 hours (Lu et al., 2017). Substrates with electron-withdrawing moieties within their structures recorded higher catalytic conversions.
On the other hand, when 1,2-epoxyethylbenzene was used as the substrate, only 72% of it was changed to products within the same duration. However, extending the reaction time to 12 hours led to the complete conversion of substrates into the corresponding cyclic carbonates apart from the electron-donating 1,2-epoxyethylbenzene.
High-pressured cycloaddition of carbon dioxide to epoxides at a pressure of 20 atmospheres and temperatures of 80 °C with the PMOF as a catalyst had better outcomes. When n-Bu4NBr was included in the reactants at a concentration of 1 mmol and 0.01 mmol of the PMOF catalyst 1 (based on V), conversion rates exceeding 90% were recorded within 4 hours. Additionally, it was easy to separate the catalyst from the reaction mix via centrifugation and filtration. Dichloromethane was used to clean the separated samples, which were then air-dried and recycled five more times without significant changes in the overall catalytic efficiency.
These findings indicated that the PMOF was stable and had good recyclability. A high catalytic capacity was also observed when the catalyst was used for the heterogeneous oxidative desulfurization of sulfides (Lu et al., 2017). The only shortcoming with this catalyst was that it required high temperatures and pressures (80oC and 20 atmospheres), as well as longer reaction times (12 hours), to produce satisfactory substrate conversion rates.
Silver Coordination Polymer
Extensive studies have been conducted on the nucleophilic addition of terminal alkynes to different unsaturated electrophiles. This reaction mechanism makes it possible to introduce the alkyne functionality (Lescouet, Chizallet, & Farrusseng, 2012). The ensuing propargylic alcohols react with carbon dioxide, which is converted into cyclic carbonates. To guarantee that this conversion occurs, it is necessary to provide catalysts made of heavy transition metals.
In addition, subsequent separation and refining of products from the reactants, catalysts, and other related materials are needed. A process that facilitates various transformations of alkynes through a sequence of distinct catalytic stages and one checkup stage is necessary for the environmentally friendly conversion of CO2 to useful chemicals. It has been shown that silver complexes can σ-activate terminal alkynes as well as π-activate internal alkynes containing C-C triple bonds for distinct reactions.
However, most of these studies have focused on homogeneous catalysis that employs large quantities of silver-based catalysts. Nonetheless, such endeavors have been challenged by low catalytic activities and leakage of active species, which have complicated the use of silver complexes to activate alkynes in the heterogeneous state.
Zhou et al. (2017) incorporated the tetrakis(4-carboxyphenyl)ethylene (H4TCPE) group as a four-point joined bulge to add alkynophilic Ag ions in the porous and coordination frameworks. It was projected that the dispersed silver chains in this structure would show immense capacity trigger at the π position of C-C triple bonds. Ag-TCPE represented an efficient catalytic potential in a heterogeneous framework for the carboxylative cyclization of propargylic alcohols with CO2 (Zhou et al., 2017). The effective catalysis of azide-alkyne cycloaddition was another indication that the silver zones had activation properties.
Functionalized IRMOF-3
Kim and Park (2013) developed a functionalized MOF named F-IRMOF-3 that possessed a quaternary ammonium group. The MOF was developed using a rapid precipitation and solvothermal technique. The system demonstrated good catalytic activity for the cycloaddition of allyl glycidyl ether and CO2 minus any solvent. The F-IRMOF-3 containing a larger alkyl chain in its structure, as well as a more nucleophilic anion, showed an enhanced activity for the cycloaddition reaction.
The catalytic activity of the F-IRMOF-3 at 120oC and carbon dioxide pressure of 1.2 MPa in a semi-batch reactor for 4 hours indicated that it was possible to recycle the catalyst three consecutive times with only a slight loss in the original catalytic activity. However, filtration (for recovery purposes) led to the loss of part of the catalyst. Overall, it was concluded that the insertion of a faulty ZnO to F-IRMOF-3 through the rapid precipitation technique was more beneficial than the traditional solvothermal approach for the cycloaddition reaction because of the acid-base bifunctional active sites.
Best Candidates of MOFs as Heterogeneous Catalysts
Even though a number of MOFs have been used as heterogeneous catalysts in the chemical conversion of carbon dioxide, it is still important to develop more effective MOFs, especially under mild reaction states. Mild reaction conditions have the advantage of reducing energy use and production costs.
Furthermore, aspects such as the framework’s affinity for carbon dioxide should be examined. Based on this literature review, the best candidates of MOFs as heterogeneous catalysts include Hafnium-based MOFs, nbo MOF-505, acylamide-containing MOFs, and ultra-stable porphyrin Zr MOF. All these were chosen based on their stability and catalytic capacity at low temperatures (room temperature on average), low carbon dioxide pressure requirements (1 atmosphere or lower), relative yield of cyclic carbonates, and the ability to be recycled without losing catalytic activity.
Summary
Carbon dioxide is a copious, inexpensive, and non-toxic, renewable source of C1. However, it has greenhouse effects, which necessitates the reduction of its concentrations in the environment. In recent times, the selective and effective transformation of light olefins with CO2 as a 1-carbon atom source into enantiomerically pure cyclic carbonates has received a lot of attention from chemists and governments.
The increased interest is due to the large economic impact of the products and the environmental consequences of carbon dioxide. Therefore, the development of effective catalytic processes that convert carbon dioxide into economically functional substances is of immense interest. For instance, cyclic carbonates are a useful group of substances that can serve as electrolytes, solvents, and intermediaries in the production of other chemical substances.
The overall synthetic process entails two discrete catalytic processes that differ in their machinery. The first step is the lopsided epoxidation of olefins, whereas the second step is the disproportionate coupling of the epoxide with carbon dioxide. The two procedures need asymmetric catalysts to guarantee enantioselectivity in addition to a successive separation and refinement process of the yield from the reactants, catalyst, and other substances.
MOFs provide effective catalysts that mediate these reactions. However, there is a need to identify more effective, and efficient, catalysts for this process. This review on the efficiency of various MOF-based catalysts shows that potential candidates for the conversion of carbon dioxide to cyclic carbonates include Hafnium-based MOFs, nbo MOF-505, acylamide-containing MOFs, and ultra-stable porphyrin Zr MOF.
References
Beyzavi, M. H., Klet, R. C., Tussupbayev, S., Borycz, J., Vermeulen, N. A., Cramer, C. J.,… Farha, O. K. (2014). A hafnium-based metal–organic framework as an efficient and multifunctional catalyst for facile CO2 fixation and regioselective and enantioretentive epoxide activation. Journal of the American Chemical Society, 136(45), 15861-15864.
Beyzavi, M. H., Stephenson, C. J., Liu, Y., Karagiaridi, O., Hupp, J. T., & Farha, O. K. (2015). Metal–organic framework-based catalysts: Chemical fixation of CO2 with epoxides leading to cyclic organic carbonates. Frontiers in Energy Research, 2(63), 1-10.
Darunte, L. A., Walton, K. S., Sholl, D. S., & Jones, C. W. (2016). CO2 capture via adsorption in amine-functionalized sorbents. Current Opinion in Chemical Engineering, 12, 82-90.
Eddaoudi, M., Sava, D. F., Eubank, J. F., Adil, K., & Guillerm, V. (2015). Zeolite-like metal–organic frameworks (ZMOFs): Design, synthesis, and properties. Chemical Society Reviews, 44(1), 228-249.
Feng, D., Chung, W. C., Wei, Z., Gu, Z. Y., Jiang, H. L., Chen, Y. P.,… Zhou, H. C. (2013). Construction of ultrastable porphyrin Zr metal–organic frameworks through linker elimination. Journal of the American Chemical Society, 135(45), 17105-17110.
Gao, W. Y., Chen, Y., Niu, Y., Williams, K., Cash, L., Perez, P. J.,… Ma, S. (2014). Crystal engineering of an nbo topology metal–organic framework for chemical fixation of CO2 under ambient conditions. Angewandte Chemie, 126(10), 2653-2657.
Gao, W. Y., Wojtas, L., & Ma, S. (2014). A porous metal–metalloporphyrin framework featuring high-density active sites for chemical fixation of CO2 under ambient conditions. Chemical Communications, 50(40), 5316-5318.
Guillerm, V., Weseliński, Ł. J., Belmabkhout, Y., Cairns, A. J., D’elia, V., Wojtas, Ł.,… Eddaoudi, M. (2014). Discovery and introduction of a (3, 18)-connected net as an ideal blueprint for the design of metal–organic frameworks. Nature Chemistry, 6(8), 673-680.
Han, Q., Qi, B., Ren, W., He, C., Niu, J., & Duan, C. (2015). Polyoxometalate-based homochiral metal-organic frameworks for tandem asymmetric transformation of cyclic carbonates from olefins. Nature Communications, 6, 1-8. Web.
Hug, S., Stegbauer, L., Oh, H., Hirscher, M., & Lotsch, B. V. (2015). Nitrogen-rich covalent triazine frameworks as high-performance platforms for selective carbon capture and storage. Chemistry of Materials, 27(23), 8001-8010.
Kim, J., Kim, S. N., Jang, H. G., Seo, G., & Ahn, W. S. (2013). CO2 cycloaddition of styrene oxide over MOF catalysts. Applied Catalysis A: General, 453, 175-180.
Kim, Y. J., & Park, D. W. (2013). Functionalized IRMOF-3: An efficient heterogeneous catalyst for the cycloaddition of allyl glycidyl ether and CO2. Journal of Nanoscience and Nanotechnology, 13(3), 2307-2312.
Kitagawa, S. (2014). Metal–organic frameworks (MOFs). Chemical Society Reviews, 43(16), 5415-5418.
Lescouet, T., Chizallet, C., & Farrusseng, D. (2012). The origin of the activity of amine‐functionalized metal–organic frameworks in the catalytic synthesis of cyclic carbonates from epoxide and CO2. ChemCatChem, 4(11), 1725-1728.
Li, P. Z., Wang, X. J., Liu, J., Lim, J. S., Zou, R., & Zhao, Y. (2016). A triazole-containing metal–organic framework as a highly effective and substrate size-dependent catalyst for CO2 conversion. Journal of the American Chemical Society, 138(7), 2142-2145.
Li, P. Z., Wang, X. J., Liu, J., Phang, H. S., Li, Y., & Zhao, Y. (2017). Highly effective carbon fixation via catalytic conversion of CO2 by an acylamide-containing metal–organic framework. Chemistry of Materials, 29(21), 9256-9261.
Liu, H., Xi, F. G., Sun, W., Yang, N. N., & Gao, E. Q. (2016). Amino-and sulfo-bifunctionalized metal–organic frameworks: One-pot tandem catalysis and the catalytic sites. Inorganic Chemistry, 55(12), 5753-5755.
Liu, J., Chen, L., Cui, H., Zhang, J., Zhang, L., & Su, C. Y. (2014). Applications of metal–organic frameworks in heterogeneous supramolecular catalysis. Chemical Society Reviews, 43(16), 6011-6061.
Lu, B. B., Yang, J., Liu, Y. Y., & Ma, J. F. (2017). A polyoxovanadate–resorcin [4] arene-based porous metal–organic framework as an efficient multifunctional catalyst for the cycloaddition of CO2 with epoxides and the selective oxidation of sulfides. Inorganic Chemistry, 56(19), 11710-11720.
Miralda, C. M., Macias, E. E., Zhu, M., Ratnasamy, P., & Carreon, M. A. (2011). Zeolitic imidazole framework-8 catalysts in the conversion of CO2 to chloropropene carbonate. ACS Catalysis, 2(1), 180-183.
Song, J., Zhang, Z., Hu, S., Wu, T., Jiang, T., & Han, B. (2009). MOF-5/n-Bu 4 NBr: An efficient catalyst system for the synthesis of cyclic carbonates from epoxides and CO2 under mild conditions. Green Chemistry, 11(7), 1031-1036.
Yang, L., Yu, L., Diao, G., Sun, M., Cheng, G., & Chen, S. (2014). Zeolitic imidazolate framework-68 as an efficient heterogeneous catalyst for chemical fixation of carbon dioxide. Journal of Molecular Catalysis A: Chemical, 392, 278-283.
Zhou, Z., He, C., Yang, L., Wang, Y., Liu, T., & Duan, C. (2017). Alkyne activation by a porous silver coordination polymer for heterogeneous catalysis of carbon dioxide cycloaddition. ACS Catalysis, 7(3), 2248-2256.
Zhu, M., Srinivas, D., Bhogeswararao, S., Ratnasamy, P., & Carreon, M. A. (2013). Catalytic activity of ZIF-8 in the synthesis of styrene carbonate from CO2 and styrene oxide. Catalysis Communications, 32, 36-40.