Abstract
Human activities continue to threaten the purity of water by releasing pollutants from industries and domestic water usage. When phosphates in aqueous solutions gain access into water bodies, they lead to the abnormal growth of algae, which reduces the concentration of dissolved oxygen. Therefore, phosphates in aqueous solutions must be reduced before the solutions are released into water bodies.
In addition, high phosphate prices necessitate techniques that enable the recycling of phosphates. One method of removing phosphates from aqueous solutions involves the formation of struvite, a complex salt made of magnesium, ammonium and phosphate. Struvite crystallization requires an alkaline pH between 8 and 10 and temperatures between 20 oC and 25 oC.
A delicate balance in the quantities of key ions such as magnesium, phosphate and ammonium is necessary for the success of the method. Therefore, the process can be modified through the addition of the deficient ions. Various studies report success of the method with varying quantities of phosphates recovered as struvite from different aqueous solutions.
The recovered struvite is commonly applied as fertilizer thereby recycling phosphates. Therefore, it can be concluded that phosphate removal through struvite crystallization is an economical and efficient recovering phosphates from aqueous solutions.
Introduction
Domestic water use and industrial activities lead to the production of aqueous solutions in the form of wastewater, which hold certain quantities of useful elements, for instance, magnesium, phosphorus and nitrogen. However, these substances have a negative impact on the aquatic environment when present at high concentrations.
Therefore, it is important to safeguard the integrity of water bodies by ensuring that these elements do not gain access into water bodies at lethal concentrations. Several techniques have been developed to reduce the amount of phosphates in aqueous solutions before they are discharged into water bodies. This paper looks at struvite crystallization as a method of phosphate removal from aqueous solutions.
Phosphate Chemistry
Le Corre et al. describe a phosphate as a salt formed from phosphoric acid [1]. Phosphates can also exist as organic compounds in the form of organophosphates, which are esters of phosphoric acid. The phosphate ion comprises five atoms, which are a phosphorus atom and four oxygen atoms arranged in a tetrahedral fashion with the phosphorus atom enclosed by the oxygen atoms.
The chemical formula of the phosphate ion is written as PO43-. The molecular weight of this radical is equivalent to 94.97 grams per mole. The creation of a phosphate compound entails the addition of an atom with a positive charge to the phosphate radical, which possesses a negative charge. As a result, an ionic complex is produced.
The precise solubility of phosphates relies on the positively charged atom that combines with the phosphate anion. For example, phosphates of alkali metals (rubidium, cesium, potassium, and sodium) as well as ammonium are soluble in water. The phosphates of other metals only show solubility to a limited extent.
Sources of Phosphates
The element phosphorus exists naturally in the form of phosphates, which are present in rocks. The pure form of phosphorus is usually obtained from mined phosphate rocks. Capdevielle et al. indicate that approximately 90% of all the mined phosphorus is used in farming as synthetic fertilizers due to the importance of phosphates to the health of flora and fauna [2].
The remaining 10% of phosphorus is used in industrial applications such as the manufacture of detergents (sodium tripolyphosphates- Na5P3O10), production of safety matches, gasoline additives, plastics, and transistors in electronic gadgets. A small fraction is used as food supplements as well as in the production of phosphate salts.
The phosphate salts are used by clinicians to treat low phosphate levels in the blood and to counter the effect of excessive blood calcium levels. Other medical uses of phosphate salts are as a laxative and antacid. Therefore, urine tends to have significant quantities of phosphates.
Most of the industrial processes release their effluents into water bodies such as lakes and rivers. These effluents often lead to detrimental effects on animals living in these water bodies. The domestic use of detergents releases phosphates into water bodies via drainage pipes, which further exacerbates the problem of phosphate pollution.
In addition, the natural process of decomposition of organic substances releases significant quantities of phosphorus into the surroundings. The naturally occurring sources of phosphates such as fluorapatite may leach and release phosphates into water bodies. Wastewater treatment plants also release substantial amounts of phosphates into the environment.
Harmful Effects of Phosphates
Surface runoff of phosphates from agricultural lands, domestic water use and industries causes the eutrophication of water bodies. US EPA explains that eutrophication arises when algae present in water bodies grow at a rate much faster than is safe for a balanced ecosystem [3].
As a result, the water body becomes clogged with algae as well as other green plants that gradually turn the water body into a swamp and ultimately into a meadow. Eutrophication also affects the animals that live in the water.
According to Mylavarapu [4], the excessive plant growth leads to a deficit of oxygen resulting in the suffocation (anoxia) of aquatic animals. In humans, excessive consumption of phosphates in food or as supplements leads to complications in patients with renal failure due to the spontaneous crystallization of phosphates as struvite hence causing bladder stones.
Removal of Phosphates
According to Le Corre et al. [1], the harmful effects of phosphates in water bodies have led to the formulation of regulations to monitor the quantities of phosphates that are allowed into water bodies. Wastewater treatment plants are usually responsible for the detoxification of aqueous solutions in the form of wastewater.
However, most of these plants receive wastewater that contains phosphates at levels that surpass the maximum allowable limits. Therefore, there is a need to reduce the quantities of phosphates in effluents from various processes before discharge into water bodies. Consequently, a number of techniques that remove phosphates have been devised. These methods include physical, biological and chemical methods.
Physical methods of phosphate removal include filtration to get rid of particulate phosphates. Filtration is efficient if a large proportion of the total suspended solids comprises phosphates. The wastewater is passed through sand or membranes.
Membranes have an added advantage of removing dissolved phosphates in addition to the particulate phosphates. Membrane bioreactors, tertiary membrane filtration and reverse osmosis structures have been developed.
Chemical methods include precipitation and crystallization. In precipitation, certain elements are added to the wastewater at different points. These elements react with phosphate ions forming insoluble phosphate salts that are removed by filtration or crystallization. Precipitation is thought to remove phosphates to levels between 0.005 and 0.04 mg per litre of wastewater.
However, the key disadvantage of precipitation is that it increases the amount of sludge produced. A novel technique is the elimination of phosphates with the formation of struvite, which is a white crystalline solid.
Struvite is a compound produced by the unprompted combination of ammonium, magnesium and phosphates in equimolar quantities. The following equation illustrates the chemical reaction that leads to struvite formation.
Mg2+ + NH4+ +HnPO43-n + 6H2O = MgNH4PO4.6H2O + nH+
Biological methods of phosphate removal include assimilation and enhanced biological phosphorus removal (EBPR). During the process of assimilation, wastewater is put in treatment ponds containing photosynthetic plants such as algae, which have huge phosphate requirements.
Wastewater is also applied to agricultural land in the course of planting seasons. EBPR uses phosphate-accumulating organisms (PAOs) that amass polyphosphates as energy stores in specialized granules. EBPR is very effective and has the capability of attaining phosphate levels as low as 0.1 milligrams in effluent.
Research Results
Struvite crystallization is a method that leads to the formation of struvite, a chemical compound consisting of magnesium, aluminium and phosphate at equal concentrations. The process was named after a German geographer Heinrich Christian Gottfried von Struve who first identified it. At optimum conditions of temperature and pH, magnesium, phosphate and ammonium ions present in an aqueous solution crystallize spontaneously to form struvite. The process can also occur naturally in animal urine and seafood.
Struvite crystallization is used as a technique for eliminating phosphate ions from aquatic solutions prior to the disposal of such solutions. The process yields struvite, which is effective as manure in the agricultural sector.
In addition, struvite crystallization is useful in the recycling of phosphates due to the escalating price of phosphates. Sensitive water purification processes such as reverse osmosis also benefit from struvite crystallization as a pre-treatment method for wastewater. The process has found immense applications in sewage and wastewater treatment plants.
Removal of Phosphorus from Landfill Leachate and the Effect of Calcium ions on Struvite Crystallization
Elimination of phosphates from an aqueous solution comprising synthetic wastewater and landfill leachate was carried out by Hassidou et al. [5] in Tunisia. The leachate was obtained from the Jbel Chakir landfill site, the biggest landfill in Tunisia that held domestic solid wastes from the greater Tunis region. Certain ions such as calcium were thought to have a significant influence on the process of struvite crystallization.
The particular aspects that encountered the effect of calcium ions were the amount, shape and clarity of the commodity recovered. Therefore, the researchers aimed at investigating the process of calcium carbonate precipitation during the elimination of phosphates with struvite removal.
The technique also intended to improve the cost efficiency of the method by shortening the continuous aeration time and determining the precise nucleation time for struvite. Condensed air was used to purge dissolved carbon dioxide gas from the solution thereby increasing the pH of the solution. Precipitation occurred when the conditions changed from acidic to alkaline.
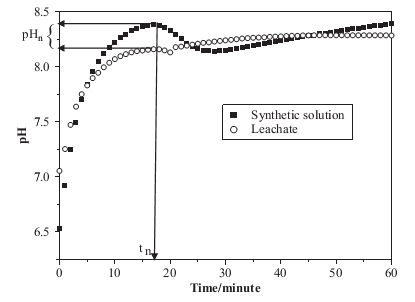
Figure 1: Changes in pH with time during phosphate removal with struvite formation and controlled degassing [5].
Three distinct steps were witnessed as depicted by figure 1. In the first phase, the pH rose rapidly due to the elimination of carbon dioxide, which lowered the acid content of the solution. A peak was attained at a pH of 8.3 followed by a steady decline (the second phase). The decline was attributed to the nucleation and enlargement of the struvite crystal.
The last phase revealed a steady increase in the pH, which was because of an increase in the carbon dioxide escaping from the system. The maximum pH (8.3) was attained after seventeen minutes implying that the nucleation time and pH for struvite formation were 8.3 and 17 minutes respectively.
The amount of soluble phosphorus that was present in the wastewater after 60 minutes was 1.5 millimoles and 2.6 millimoles in the leachate.
Hassidou et al. [5] indicated that at the end of the run, the efficiency of phosphate removal was 61.19% in the leachate and 77.61 in synthetic wastewater. Those findings implied that impurities such as calcium ions interfered with the process of crystallization and the efficiency of the technique.
Removal of Phosphates from an Aqueous Solution from Animal Manure by Struvite Crystallization alongside Carbon Dioxide Degassing
Zhang et al. [6] used a continuous U-shaped reactor in the removal of phosphates from an aqueous solution from animal manure. The reactor comprised three distinct zones namely the aeration crystallization region (1 dm3), the region of crystal growth (0.5 dm3) and the zone of crystal and supernatant separation (0.5 dm3).
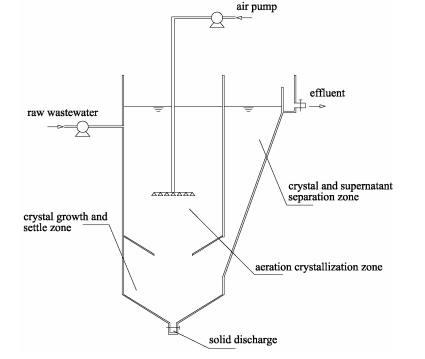
Figure 2: Continuous U-shaped reactor arrangement [6].
It was realized that the amount of phosphates obtained was lower (between 47% and 53%) when struvite was allowed to form spontaneously than when the wastewater was supplemented with magnesium (as MgCl2 at 57.5 mg per liter). The addition of magnesium yielded a phosphate recovery ratio of between 80% and 86%.
Additionally, preformed struvite was the most effective seeding material compared to sand and stainless steel. These outcomes differed from the efficiency obtained by Hassidou et al. [5] in a similar degassing technique. However, the pH at which nucleation began was almost similar in both studies. A water extraction test revealed that the struvite obtained was very useful as a slow release fertilizer.
Struvite Precipitation in Combination with Reverse Osmosis
Sewage sludge was mostly treated by anaerobic digestion with biogas production. Karabegovic et al. [7] reported that the process of anaerobic digestion reduced the organic content of wastewater since the microbes involved often consumed macromolecules such as fats, proteins and carbohydrates.
Approximately an eighth of the organic matter in wastewater was used up while a tenth was transformed into composite organic substances. The residual tenth was part of the post-digestion liquor and was accountable for the high chemical oxygen demand (COD) of such fluids.
Reverse osmosis (RO) was an effective technique of ridding the post-digestion liquor of contaminants. However, its efficacy was marred by high concentrations of ammonium ions that could not be contained by the RO membranes.
Bohdziewicz and Kuglarz [8] used struvite accumulation as a pretreatment technique of post-digestion liquors prior to reverse osmosis. Struvite was removed at a temperature of 20 oC and pH of between 9.0 and 9.5, which was significantly higher than the pH used by Hassidou et al. [5], Zhang et al. [6] and Harrison et al. [9].
It was realized that the amount of ammonium ions surpassed the stoichiometric ratio required to form struvite while the concentrations of phosphate and magnesium were less than the needed amount. Therefore, Bohdziewicz and Kuglarz [8] used phosphoric acid as well as a combination of the oxide and chloride of magnesium to amend the concentrations of magnesium and phosphate ions before the crystallization.
It was reported that the technique achieved a phosphate removal rate of 87% to 92% before the adjustment of the concentrations of magnesium and phosphorus. Conversely, a phosphate removal rate of between 98% and 99% was attained when the ratios were adjusted. The removal of COD was also affected by the form in which the magnesium ions were added.
Magnesium in the form of MgO led to a higher COD removal value than the addition of Mg as MgCl2. That observation was attributed to the traits of Mg(OH)2 that led to the precipitation of dissolved organic compounds alongside struvite. The efficiency of reverse osmosis was higher following the pretreatment of post-digestion liquor by struvite removal.
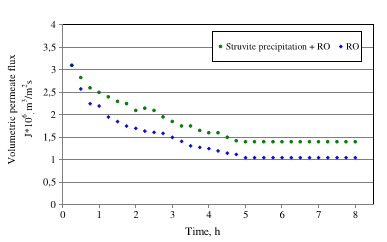
Figure 3: The effect of pretreatment of post-digestion liquor by struvite precipitation on reverse osmosis [8].
Continuous Reaction Crystallization of Struvite from an Aqueous Solution Containing
Phosphate (V) and Nitrate (V) ions
Hutnik et al. [10] investigated the removal of phosphates from solutions that contained between 0.2% and 1% of phosphates by mass and 0.0443% to 0.866% nitrate (V) ions by mass. The processes were performed in two different phases. A continuous struvite reaction crystallization unit was operated with the concentrations of magnesium, phosphate and ammonium at stoichiometric proportions.
The second phase involved a DT MSMPR crystallizer (Draft Tube, Mixed Suspension Mixed Product Removal) where magnesium ions were in excess by approximately 20%.
The temperatures in both phases were 25 oC and the pH ranged from 9 to 11. In addition, crystallization was allowed to take place for durations between 15 minutes and one hour. Hutnik et al. [10] investigated the effect of pH and the ions on the quality of struvite crystals formed.
Hutnik et al. [10] observed that as the concentration of nitrates in the aqueous solution was increased, the average size of the crystals decreased by approximately ca. 29%. In addition, there was no uniformity in the size of crystals formed. On the other hand, Hutnik et al. [10] realized that elevating the pH had a negative effect on the quality of struvite crystals formed as smaller irregular crystals were formed.
It was also noted that a reduction in the concentration of phosphate ions caused an increase in the crystal size by about ca. 23%. Longer reaction times led to an increase in the size and uniformity of the crystals formed. Moreover, the elevation of the concentration of magnesium ions was found to favor struvite crystallization leading to the formation of large tubular crystals.
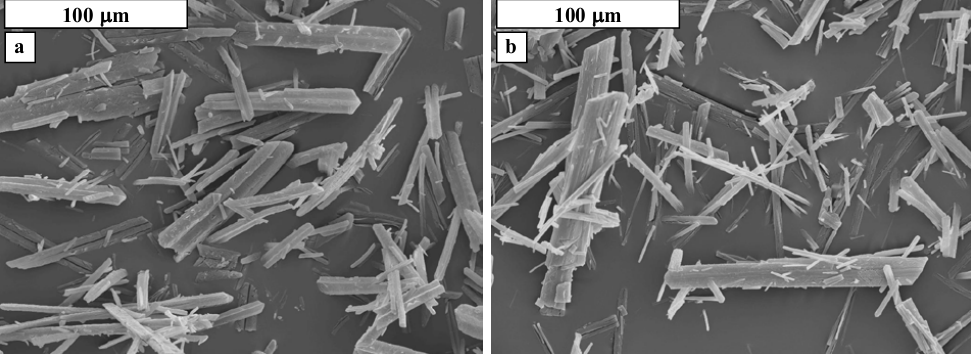
Figure 4: Scanning electron microscope images of the crystals obtained from crystallization using 0.0886% of nitrates (a) and 0.443% of nitrates (b) [10].
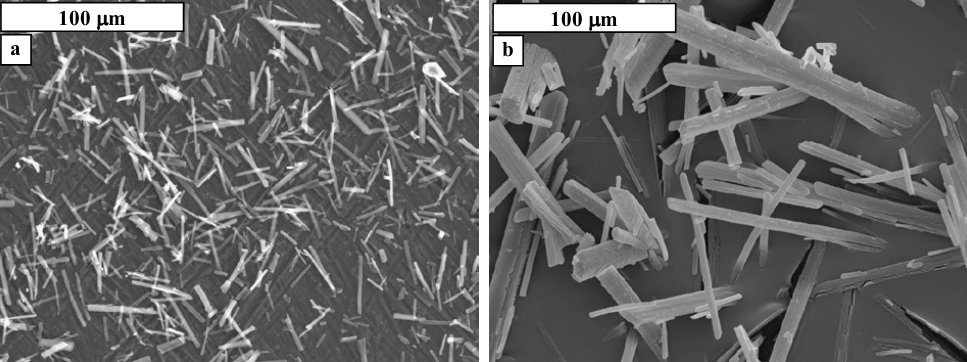
Figure 5: Scanning electron microscope images showing the effect of pH and reaction time on the size of crystal formed. In (a), the conditions were pH 11 and a reaction time of 15 minutes, whereas in (b) the conditions were pH 9 and a reaction time of one hour. The concentration of nitrate (V) ions in both cases was 0.0886% [10].
Struvite Crystallization from Diluted Aqueous Solution of Phosphate (V) Ions in the Presence of Magnesium Ions Excess
Kozik et al. [11] investigated the crystallization of struvite from an aqueous solution containing phosphate (V) ions at a concentration of 0.2 % by weight. A DT MSMPR type crystallizer was used at a temperature of 25 oC and a pH range from 8.5 to 10. Additionally, the concentration of magnesium ions surpassed the stoichiometric ratio for struvite formation by a fifth.
The average residence time for the crystallization process was between 900 and 3600 seconds. Kozik et al. [11] reported that crystals of average sizes ranging from ca. 19 to ca. 3 were obtained. A pH of 8.5 for 3600 seconds yielded decently sized crystals with satisfactory homogeneity. Kozik et al. [11] reported that at those conditions, the rate of linear growth of the crystals was between 3.62 x 10-9 and 1.68 x 10-8 metres per second.
The presence of excess magnesium ions during struvite crystallization improved the production of struvite. However, the quality of the crystals was somehow affected.
Kozik et al. [11] reported that the concentration of phosphate ions in the original aqueous solution decreased from 0.2% by weight to 9-92 milligrams per kilogram of the aqueous solution implying that the process was effective in recovering phosphates from aqueous solutions. It was noted that the optimum crystallization was obtained at the same reaction conditions as those reported by Hutnik et al. [10].
Phosphate Removal by Struvite Recovery in a Microbial Electrolysis Cell
Cusick and Logan reported that microbial electrolysis cells offered the most cost-effective way of struvite recovery from aqueous solutions [12]. Uninterrupted chemical supplementation and blower operations significantly increased the cost of struvite by approximately 400 dollars per ton of struvite recovered compared to about 50 dollars spent on production of phosphorus from phosphate deposits.
The quantities of chemicals added contributed to about 97% of the total amount spent on struvite precipitation. Therefore, it was necessary to minimize the costs of struvite formation from wastewater to make the entire process economically viable. Microbial electrolysis cells simultaneously produced hydrogen gas and removed phosphates from wastewater without the need for chemical addition and large energy inputs.
The two types of cathodes used by Cusick and Logan were mesh and plate cathodes both made of stainless steel. Struvite recovery rates ranging from 20% to 40% were obtained, which were significantly lower than the values reported by Hassidou et al. [5], Zhang et al. [6] and Harrison et al. [9].
However, the process was more efficient (73%) than the conventional struvite recovery procedures in terms of energy requirements. It was realized that net cathodes were more efficient than the flat cathodes. It was concluded that additional research and optimization of the microbial electrochemical cells could save a substantial amount of energy during phosphate removal from wastewater.
The Effect of Temperature, Reaction Time, and pH on the Amount of Phosphates Recovered as Struvite from Aqueous Solutions
Ahmad and Idris [13] investigated the parameters that affected the process of struvite crystallization during phosphate removal. Aerobically digested wastewater was used in the investigation. Temperatures higher than 100 oC were applied to sludge following the treatment of wastewater.
The amount of soluble phosphates was quantified in the wastewater as the heating temperature rose. Ahmad and Idris realized that the amount of phosphorus released into the wastewater increased with an increase in temperature and time of heating [13].
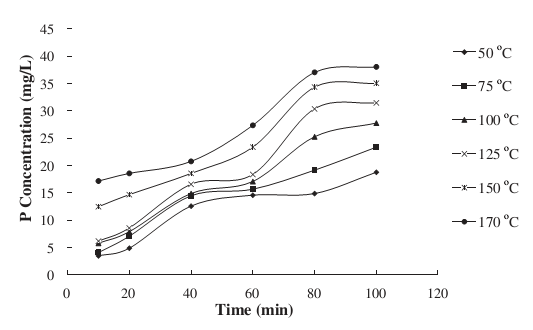
Figure 6: The influence of temperature on the quantities of dissolved phosphorus [13].
The concentration of dissolved and suspended phosphorus was 3.4 and 4.6 mg per liter prior to heating. However, the highest amount of liberated phosphorus was attained following heating at 175 oC for 100 minutes. The release of phosphorus with an increase in temperature was attributed to microbial cells whose membranes were disrupted following heating leading to the release of phosphorus.
It was also realized that more phosphorus was released into the wastewater at low pH. Ahmad and Idris attained the highest levels of phosphorus release were attained at pH of 2 (48.9 milligrams per liter) and pH of 4 (48.6 milligrams per liter) [13]. The reason for that occurrence was that the majority of phosphates in wastewater sludge were organophosphates that were easily liberated by heating.
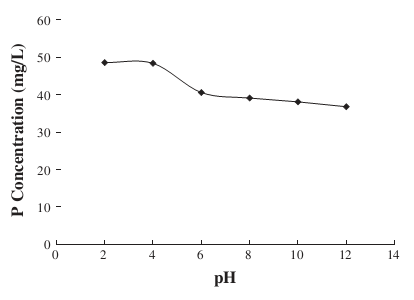
Figure 7: Influence of pH on the release of phosphorus [13].
A separate investigation by Yariv and Holger investigated the prospects of raising the pH of aqueous solutions by exclusion of carbon dioxide and using gases such as plain air, oxygen and nitrogen to replace the vacuum left by CO2 [14]. The pH of the solution was monitored by a computer-regulated setup. Yariv and Holger [14] observed that plain air eliminated carbon dioxide leading to an increase in pH up to 8.53.
Pure nitrogen, conversely, elevated the pH of the solution to 10.4 after one day. A separate trial where carbon dioxide was eliminated by precipitation as calcium carbonate increased the pH to about 9.4.
All these pH values were within the optimum range required for struvite precipitation. Yariv and Holger [14] concluded that it was possible to reduce the costs of struvite precipitation by using air instead of chemicals to raise the pH of aqueous solutions.
The Effect of Copper (II) Ions on the Quality of Struvite Produced
It was thought that impurities affected the crystallization of struvite. Therefore, Hutnik et al. [15] sought to determine the effect of copper ions in aqueous solutions on struvite crystallization. An aqueous solution containing 1 to 2% of phosphate ions by mass and 0.5 milligrams of copper ions per kilogram of solution was used.
Struvite crystallization was performed at stoichiometric conditions and magnesium ion excess. Hutnik et al. [15] noted that the presence of copper ions in the reactor increased the size of struvite crystals by 6% at stoichiometric conditions. However, lowering the phosphate concentration and increasing the quantity of magnesium yielded 9% to 13% larger crystals.
Table 1: The effect of copper ions of struvite crystals at a temperature of 25 oC, pH 9 and mean residence time of 900 seconds [15].
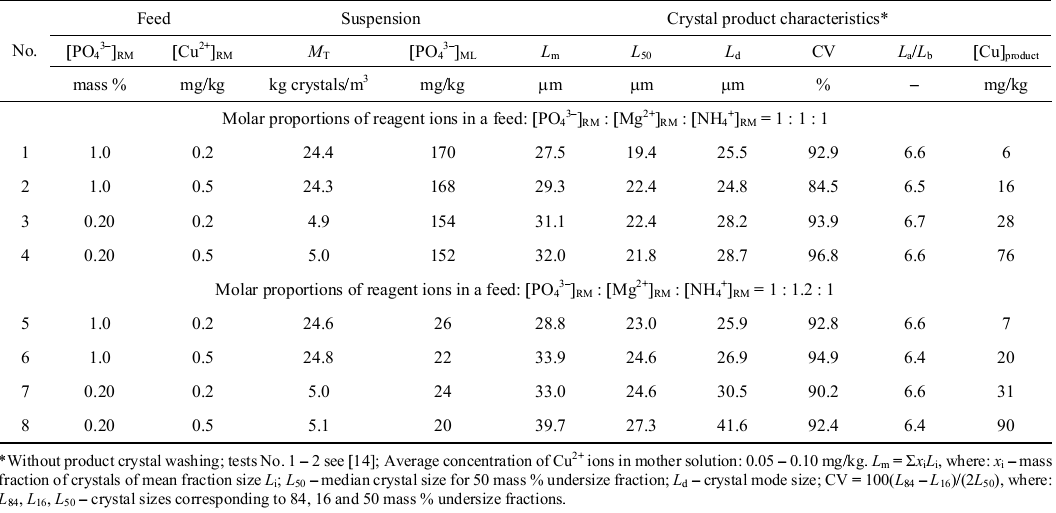
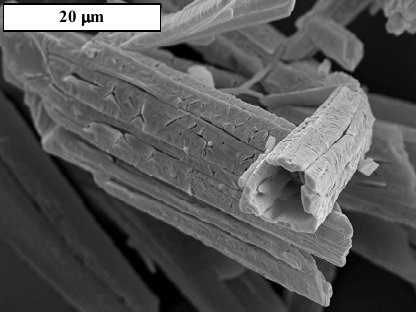
Figure 8: The morphology of struvite crystals produced in the presence of copper (II) ions [15].
Analysis of the final product revealed the presence of copper (II) hydroxide at concentrations ranging from 6 to 90 milligrams per kilogram. It was concluded that the presence of copper ions promoted the formation of tubular struvite crystals.
Struvite Formation from Human Urine
Etter et al. [16] investigated the possibility of using human urine to concentrate phosphates for agricultural use. Urine used in the study was collected from Siddhipur village in Nepal because of the common practice of the creation of urine separation pits in the community. Magnesium oxide from a local magnesite was used as a magnesium source.
Etter et al. [16] reported that more than 90% of phosphates was recovered from the reactor with an external filtration unit with minimal usage of magnesium oxide (1.1 moles of magnesium per 1 mole of phosphate) in one hour.
Rough nylon filters were used in the filtration unit. Conversely, a separate sedimentation setup yielded only 40% of phosphates. Etter et al. [16] concluded that large volumes of urine were needed for the process to yield substantial volumes of struvite.
The designing of struvite crystallization machinery must put into consideration factors such as the growth rate of the struvite crystals and the supersaturation of the aqueous solutions. For this reason, several studies have been carried out to model the process of struvite recovery. Harrison et al. [9] focused their attention on the dynamics of struvite crystallization with the aid of a computer package referred to as MINTEQA2.
Harrison et al. [9] reported that the software determined struvite as being insoluble at moderately alkaline pH and that temperature did not have a substantial effect on the solubility of struvite. Those findings implied that optimum crystallization was supposed to take place at moderately basic conditions.
The formation of large crystals implied that more of the dissolved struvite was incorporated into the crystals hence translating to higher struvite recovery rates from the solutions. However, despite those observations, various studies continued to obtain varying quantities of struvite from wastewater and other aqueous solutions.
For example, Zhang et al. [6] reported improved efficiencies when magnesium was supplemented (80-86% recovery rates) compared to 47-53% recovery rates when crystallization was allowed to take place spontaneously without magnesium supplementation. In both instances, Zhang et al. [6] used temperatures of 25 oC and the pH range described by Harrison et al. [9].
Conversely, Hassidou et al. [5] obtained phosphate removal rates of 61.19% from leachate and 77.61% from synthetic wastewater. Otter et al. [16] were able to recover up to 90% phosphates from human urine. These variations in struvite recovery rates suggested that there were certain aspects in the compositions of the aqueous solutions that altered the struvite recovery rates.
The contentious issue, therefore, became the optimum conditions for the most efficient removal of phosphates from aqueous solutions. It was hypothesized that factors other than temperature and pH affected the kinetics and efficacy of struvite crystallization. Therefore, there was a need to ascertain the precise factors and their consequences on struvite crystallization.
Hutnik et al. [10], Kozik et al., [11] and Hutnik et al. [15] looked at the efficiency of the formation of struvite crystals under varying concentrations of various ions such as nitrates phosphates and magnesium. Hutnik et al. [10] and Kozik et al. [11] noted that better crystallization rates were obtained with magnesium concentrations that surpassed the stoichiometric concentrations in struvite.
Zhang et al. [6] observed that higher struvite yields corresponding to higher phosphate removal rates were attained with magnesium supplementation. These observations deduced that in spite of struvite formation occurring at the stoichiometric concentrations of magnesium, ammonium and phosphates, an excess of magnesium ions enhanced the process of crystallization.
The effect of interfering ions on struvite crystallization was also investigated by Ahmad and Idris [13] and Hutnik et al. [15]. Ahmad and Idris [13] observed that calcium ions acted as impurities and lowered struvite crystallization rates.
These observations suggested that the presence of other cations in aqueous solutions was likely to lower struvite crystallization. On the other hand, contrary to the expectations, Hutnik et al. [15] realized that copper ions augmented struvite crystallization and even led to the formation of large crystal sizes.
Conclusion
From all the papers reviewed in this research, it was evident that struvite formation occurred at alkaline pH between 8 and 10 and temperatures between 20 oC and 25 oC. The optimum pH was attained by the removal of carbon dioxide gas. The resultant struvite was useful as a slow release manure, which did not require additional processing apart from washing and drying.
The conditions in the reacting vessels were adjusted to optimize struvite formation, for example, through the addition of magnesium ions to ensure that the reactants were in the right proportions according to the molecular formula of struvite.
It was noted that adding magnesium as magnesium oxide led to lower COD values than when it was added as magnesium chloride. However, Capdevielle et al. [2] and Capdevielle et al. [17] noted that magnesium oxide significantly increased the amount of struvite recovered. Overall, excessive magnesium ions led to better rates of struvite crystallization.
The presence of interfering cations such as calcium ions lowered the efficacy of crystallization while copper ions augmented crystallization. From these findings, it appeared as though there could be additional undiscovered factors that affected struvite crystallization.
Therefore, further investigations could be carried out to establish the additional factors that influenced phosphate removal from aqueous solutions through struvite crystallization.
Struvite precipitation was also used as a pretreatment technique to improve the efficiency of other purification methods such as reverse osmosis. Therefore, this research concluded that the removal of phosphate from aqueous solutions via struvite formation was an efficient method of preventing the pollution of water bodies and recycling phosphates.
References
[1] K. S. Le Corre, E. Valsami-Jones, P. Hobbs, and S. A. Parsons, “Phosphorus Recovery from Wastewater by Struvite Crystallization: A Review.” Critical Reviews in Environmental Science and Technology, vol. 39 no. 2009, pp. 433–477, May 2009.
[2] A. Capdevielle, E. Sýkorová, F. Béline, and M. Daumer, “Kinetics of struvite precipitation in synthetic biologically treated swine wastewaters.” Environmental Technology, vol. 35, no. 10, pp. 1250-1260, Jan. 2014.
[3] US EPA. “Nutrient pollution.” Internet: https://www.epa.gov/nutrientpollution/issue, Mar. 16 2014 [May 18 2014].
[4] R. Mylavarapu. “Impact of phosphorus on water quality.” Internet: http://edis.ifas.ufl.edu/ss490, Apr. 14 2014 [May 18 2014].
[5] S. Hassidou, T. Ismail and B. A. Mohamed, “Phosphorous removal from Tunisian landfill leachate through struvite precipitation under controlled degassing technique.” Desalination and Water Treatment, vol. 21, no. 2010, pp. 295–302, Sep. 2010.
[6] T. Zhang, P. Li, C. Fang, and R. Jiang, “Phosphate recovery from animal manure wastewater by struvite crystallization and CO2 degasification reactor.” Ecological Chemical Engineering Science, vol. 21, no. 1, pp. 89-99, 2014.
[7] L. Karabegovic, M. Uldal, A. Werker and F. Morgan-Sagastume, “Phosphorus recovery potential from a waste stream with high organic and nutrient contents via struvite precipitation,” Environmental Technology, vol. 34, no. 7, pp. 871-883, Aug. 2013.
[8] J. Bohdziewicz and M. Kuglarz, “Treatment of post-digestion liquors with the application of struvite precipitation and reverse osmosis.” Desalination and Water Treatment, vol. 5, no. 2013, pp. 366–373, Jan. 2013.
[9] M. L. Harrison, M. R. Johns, E. T. White, and C. M. Mehta, “Growth rate kinetics for struvite crystallization.” Chemical Engineering Transactions, vol. 25, no. 2011, pp. 309-314, 2011.
[10] N. Hutnik, B. Wierzbowska, K. Piotrowski, and A. Matynia. “Continuous reaction crystallization of struvite from solution containing phosphate (V) and nitrate (V) ions.” The Online Journal of Science and Technology, vol. 3, no. 2, pp. 68-66, Apr. 2013.
[11] A. Kozik, H. Nina, P. Krzysztof, and M. Andrzej. “Continuous reaction crystallization of struvite from diluted aqueous solution of phosphate(V) ions in the presence of magnesium ions excess.” Chemical Engineering Research & Design, vol. 92, no. 3, pp. 481-489, Mar. 2014.
[12] R. D. Cusick and B. E. Logan, “Phosphate recovery as struvite within a single chamber microbial electrolysis cell.” Bioresource Technology, vol. 107, no. 2012, pp. 110-115, 2012.
[13] A. A. Ahmad and A. Idris, “Release and recovery of phosphorus from wastewater treatment sludge via struvite precipitation.” Desalination and Water Treatment, vol. 2013, pp. 1-8, Jun. 2013.
[14] C. Yariv and K. Holger, “Increasing the pH of wastewater to high levels with different gases- CO2 stripping.” Water, Air and Soil Pollution, vol. 159, no.2004, pp. 265-275, Jun. 2004.
[15] N. Hutnik, B. Wierzbowska, K. Piotrowski, and A. Matynia. “Effect of copper (II) ions on quality of struvite produced in continuous reaction crystallization process at the magnesium ions excess.” Advances in Chemical Engineering and Science, vol. 2013, no. 3, pp. 1-6, May. 2013.
[16] B. Etter, E. Tilley, R. Khadka, and K. M. Udert “Low-cost struvite production using source-separated urine in Nepal.” Water Research, vol. 45, no. 2, pp. 852-862, Jan. 2011.
[17] A. Capdevielle, E. Sykorová, B. Biscans, F. Béline, and M. Daumer, “Optimization of struvite precipitation in synthetic biologically treated swine wastewater—Determination of the optimal process parameters.” Journal of Hazardous Materials, vol. 244-245, no. 2013, pp. 357-369, 2013.