Introduction
Respiration is step-wise oxidation of reduced carbon compounds either by oxygen in aerobic organisms or by some oxidized compounds like NO3–, SO42- in the case of anaerobes. While some of the intrinsic energy stored in these compounds is released as heat, the remainder is stored in the form of ATP, produced through oxidative phosphorylation. The first steps of both anaerobic and aerobic respiration involve a series of sequential reactions referred to as glycolysis (Embden-Meyerhof-Parnas pathway), in which complex carbohydrates are first converted to simple monosaccharides, viz. glucose and fructose, which are oxidized to two molecules of pyruvate. Further catabolism of pyruvate by Kreb’s cycle produces CO2 and reducing equivalents like NADH + H+, which feed electrons to oxidative electron transport chain for complete oxidation, and in turn, generates ATP. About 36 molecules of ATP are produced from one mole of glucose. In the absence of an electron acceptor like O2, several strict or facultative anaerobic micro-organisms carry out incomplete oxidation of carbohydrates by fermentation. Here, ATP is produced through substrate-level phosphorylation involving the steps of glycolysis up to pyruvate, which is then converted to the end products like ethanol and CO2 or lactate or acetate. The net gain of ATP per mole of glucose in glycolytic fermentation is just two. Fermentation executes an important redox balance in the cells. During glycolysis, two moles of NADH + H+ are produced, and in absence of an electron acceptor, there is a possibility that the oxidized NAD+ pool gets exhausted. Fermentation ensures the recycling of this NAD+ from its reduced form. The yeasts are classified under the Ascomycetes group of fungi, which under anaerobic conditions carry out alcoholic fermentation by the following reaction:
C6H12O6 → 2C2H5OH + 2CO2
They are unicellular, spherical, oval cells dividing by budding, but under certain conditions they also produce filaments. Some yeasts also exhibit sexual reproduction by fusion followed then by budding. The common habitats of yeasts are fruits, flowers, and bark. The morphological and biochemical features of yeasts have been documented by Madigan, Martinko, and Parker (2000). The most important commercial yeasts are Baker’s and Brewer’s yeasts, members of the Saccharomyces cerevisiae, whose genome is completely sequenced. Owing to the energy crisis, ethanol production from yeast has gained immense importance because ethanol can blend with diesel and petrol. In commercial ethanol production, the non-tolerance of yeast cells towards high sugar and ethanol concentrations is a major constrain. Another factor that influences ethanol production is the inoculum size. In reactors, after a particular cell density is attained, growth slows down and ethanol starts to produce. If starting cell density is high, it takes much less time to ferment and higher ethanol is produced in less time. Conversely, ethanol yield in low-density inoculum as starting material would be poor and delayed. High cell density has also relevance in terms of tolerance towards high sugar/ethanol concentrations per unit biomass Nevertheless, such variation in initial cell density has not much effect on either sugar/ethanol tolerance or the conversion efficiency of sugar to ethanol. It becomes desirable, therefore, to optimize the inoculum size suitable for the highest ethanol production in the least time. It was hypothesized that by increasing yeast concentration the net ethanol production and CO2 evolution, and their rates of production would increase. For this, we have monitored CO2 production with glucose in presence of two different concentrations of yeast cells.
Methods
The rate of fermentation can be estimated either by monitoring the production of ethanol or CO2. The latter is a convenient method as many “in-house” fermentation apparatus has been fabricated with an objective to measure CO2 evolution from the reaction mixtures comprising Baker’s yeast cells, grown in a suitable medium or obtained from a commercial source (food additive), and a source of sugar generally glucose. CO2 evolution is routinely measured by displacement of aqueous phase by CO2 in the graduated pipette, by upward movement of water bubble in a graduated pipette, or by Durham tubes inversely suspended in reaction mixtures. In the present experiment, four test (fermentation) tubes were placed in the water contained in four flasks.
These flasks were equilibrated for constant temperature by suspending in a water bath set at 30oC. The four labeled fermentation tubes were filled with reaction mixtures containing: (#1) 3 ml of sugar (glucose) solution (usual concentration is 2-10%), (#2) 1 ml of yeast suspension usually prepared by homogenizing 1 packet dry Baker’s yeast in 200 ml distilled water, (#3) glucose solution + yeast suspension and (#4) glucose solution + three times the yeast suspension (3 ml). In all the tubes the final volume was adjusted to 7 ml by adding distilled water and all the contents were thoroughly mixed. The fermentation tubes were attached to 1 ml graduated pipettes whose ends were fitted with aquarium tubes whose ends were kept open for suction by a pipette pump. After a pre-incubation of 5 min at 30oC, the contents of the tubes were sucked to ca. zero marks of the pipettes and the ends of the tubes were clamped air-tight to ensure that the liquid does not run down. In such assembly, if gas is produced, the liquid level would fall in the pipettes, and the difference would be equal to the volume of gas. The tubes were incubated for 20 min and at every 2 min interval, the change in volume of the liquid in pipettes was recorded. A tabular representation of the measured values (final minus the initial) clearly gave an idea of whether CO2 was released, and if so, then in which reaction mixture(s). The tabular values in the Microsoft Excel® spreadsheet were plotted using the same software. Using a line diagram, the time-kinetic evolution of CO2 was represented for each of the four reaction mixtures. The final absolute values of CO2 evolution in 20 min were indicated at the end of the lines. As many students have carried out the same experiment, in which one variable that is increasing in inoculum size was tested, the values were averaged and standard deviation was calculated for the pooled data. The values falling beyond ±10% from the mean were discarded.
Results
The time-dependent evolution in the four sets were first tabulated in Table 1 and then these values were plotted as line diagrams (Fig. 1). Appropriate table captions and figure legends were included. Legible symbols and proper details were given for each set. The following data were obtained:
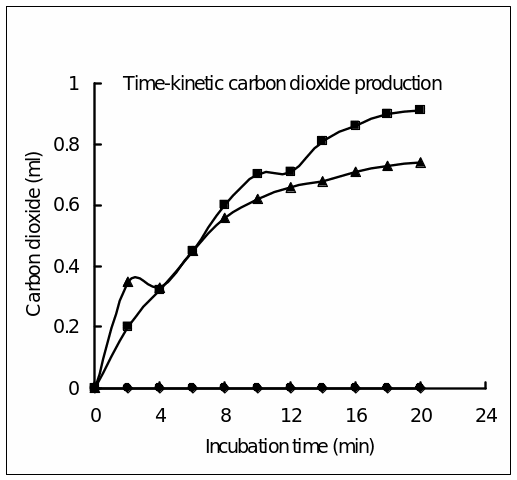
Discussion
In tubes #1 and 2, in which sugar or yeast inoculum alone was kept there was no evolution of CO2 recorded. It makes sense because yeast cells incubated without substrate, or substrate without the organism would not carry out fermentation. These tubes were considered and minus substrate and minus enzyme controls. The volumes were adjusted with water so that the final concentrations of yeast cells and sugar do not vary between sets. In tube #3, in which 1 ml of yeast suspension and sugar were simultaneously added, CO2 started to evolve from the beginning, but the rates slowed down after 8 min. Overall, the rate of CO2 production was 2.22 ml h-1. In tube #4, in which yeast concentration was tripled, the CO2 evolution rates were higher than in tube #3, but it slowed down after 10-12 min. Here, the CO2 production rate was calculated to be slightly higher (2.73 ml h-1). Obviously, this difference in the rates was attributed to the difference in the yeast concentration. But the increase in evolution rates was not proportionate to the increase in inoculum size. This suggests that most of the cells in 3x yeast suspension were not fermenting. It would have been appropriate to gradually increase the concentration rather than to bring it to 3-fold. In the given condition, 1x yeast concentration seems to be sufficient to give optimal CO2 evolution. In another investigation (Spilatro, 2000), the CO2 evolution rate by yeast fermentation was found to be 5.4 ml h-1.
Given the difference in strains used and conditions applied, the rates recorded here are corresponding to the previously reported values. For e.g. calcium stimulates the fermentation process and was not added in present assays. In an attempt to roughly estimate the ethanol production rates in molar terms, the volume of CO2 was converted to its molar concentration using the formula: P1V1/T1 = P2V2/T2. For this, CO2 volume was extrapolated at absolute temperature, assuming that the pressure of 1 atmosphere was maintained in the pipettes. 0.74 ml in tube #3 at assay temperature, 273 + 30 = 303 K would correspond to 0.67 ml at 273 K [(0.74 x 273)/303]. Likewise, for tube #4 the corresponding value of 0.91 ml would be 0.82 ml at absolute temperature. At normal temperature and pressure, 1 mole of gas occupies 22,700 ml. This means 0.67 ml and 0.82 ml of CO2 would be equivalent to 29.5 and 36.1 µmole, respectively. In other words, approximately these many moles were produced in the two tubes (#3 and 4) after 20 min of incubation. Since CO2 is produced equimolar to ethanol in glucose fermentation, we predict 29.5 and 36.1 µmole of ethanol produced in 20 min in tubes #3 and 4, respectively. Thus the net increase in extrapolated ethanol concentration was only 120% while there was a 300% increase in the yeast cells. These results corroborate the earlier findings that high inoculum size does not significantly affect the rate of ethanol production (Pramanik, 2003). Our results indicate that rather than abruptly increasing the yeast cells, it would be better to gradually raise the cell number and monitor CO2 production so that effective inoculum size can be deduced that gives the highest ethanol production in unit time. It is also necessary to include variables like different sugar and calcium concentrations at optimal yeast concentration, and use controls in which live cells are replaced by heat-killed cells. To sum up, the experiment did indicate a positive role of inoculum size on CO2 evolution as hypothesized, but there was no quantitative relationship between these two variables.
References
Madigan, Michael T., John M. Martinko, and Jack Parker. Brock Biology of Microorganisms, Ninth Ed. New Jersey: Prentice-Hall Inc., 2000.
Pramanik, K. “Parametric Studies on Batch Alcohol Fermentation Using Saccharomyces Yeast Extracted from Toddy.” Journal of Chinese Institute of the Chemical Engineers 34, 4 (2003): 487-492.
Spilatro, Steven R. “Yeast on the Rise: Investigative Study of Fermentation in the Introductory Biology Curriculum.” Marietta College Biology Department. 2000. Web.