The main gases found in blood are carbon dioxide, oxygen and nitrogen (Bothara, 2007). The blood found in arteries is richer in oxygen and nitrogen while the one found in veins is richer in carbon dioxide. There are some arguments that blood gases exist freely while others view that such gases exist in a frail chemical combination.
As such, certain amount of carbonic acid that exists in chemical nature is found in bicarbonate of soda and the rest forms a base with phosphate. Blood gases refer to quantification of the amount of carbon dioxide and oxygen available in blood (Toffalleti, 2001). In addition, it is used to quantify the level of blood acidity.
Currently, there are various methods of measuring blood gases and each strategy has its perceived merits and demerits (Notes n.d.). ‘Mallinckrodt Sensor System’ employs a conservative “blood-gas technology” and it gives the results within ninety seconds. However, this method cannot allow a continuous blood assessment.
Biomedical sensors use ocular quantification methodologies based on illumination from fibre optic bundle across a PH receptive dye. The light generated is returned to the apparatus through a different fibre optic bundle. Nevertheless, this method has a restricted accuracy and it takes more time to react.
CDI 3M Healthcare utilizes dyes that incandesce at various intensities of carbon dioxide, oxygen and PH. It has been argued that this method is less accurate as it needs intervallic convection blood gases for verification (Ricco, 1997).
Pulsed optical fluorescence is another method used in assessment of blood gases. An illumination of specific color intensity is passed through a fibre optic cable and then to a sensor that measures blood gases. In addition, calorimetric carbon dioxide detectors are used in blood gases test. There are those which depend on the acidity nature of carbonic acid and those which depend on ocular assessment techniques.
Mass spectrometer techniques of blood gases can provide a progressive way of assessing oxygen tension (Ricco, 1997). These techniques recognize compounds according to their atomic mass. Another test used in blood gases is “transcutaneous oxygen measurement” which utilizes an electrode placed on the skin to assess oxygen tension in artery blood (Notes n.d.).
Moreover, there are traditional ‘blood gas electrodes’ such “miniaturized polarographic electrode” (fig. 1) that can be used to assess blood gases. According to Notes (n.d.), this strategy uses a “platinum cathode and a silver/silver chloride anode immersed in an electrolyte solution of potassium chloride”.
A current is then passed through the solution and an oxidation-reduction reaction occurs depending on the amount of oxygen present (Toffelli, 2001). This method has a limitation in that platinum cathode can cause protein coagulation if openly put in blood.
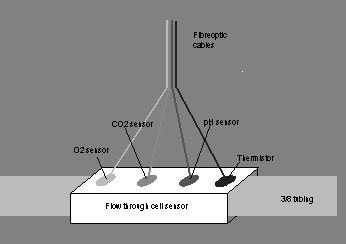
The above illustrated methods have a problem of calibration, reaction duration and assessment of blood flow. As such, these techniques may be defective and misleading. Thus, “Bentley Gas-STAT” method (Optical fluorescence microsensing) (fig. 2) may be appropriate (Notes n.d.). The method has its own calibration machine for primary calibration and this makes it effective to operate.
Furthermore, assessment values can be shown at normal blood temperature. The method also provides prompt, progressive and exact readings. In this technique, illumination of a specific color is passed through the fibre optic cable and then to the disposable sensors (Notes n.d.). These sensors assess blood gases via a membrane that allow passage of hydrogen ions and gases.
Sensors contain substances that are receptive to varying levels of carbon dioxide, oxygen and pH. When illumination produced from the apparatus arrives at the fluorescent substances on the sensor, molecules get stimulated and produce illumination of varying colors (Hasan, 2008). The illumination is transmitted back to the apparatus via a different fibre cable.
The variation in intensity of emitted and received illumination is transformed into arithmetical data (in mmHg for carbon dioxide and oxygen and pH values). This method is widely used in measurement of blood gases “in the extracorporeal circuit during bypass surgery” (Notes n.d.). This is attributed to the fact that its sensor is highly optimized and can provide a steady predictable illumination.
Sensor optimization was achieved by the use of “Taguchi double-signal optimization technique” due to increased needs for optimization during operations (Taguchi, Chowdhury and Taguchi, 2000). In this process, chemical variables were optimized since this technique is based on ‘chemical fluorescence principle’.
There are various methods of assessing blood gases with each strategy having its merits and demerits. In line methods have disadvantages in calibration, reaction time and authenticity of their results. However, optical fluorescence microsensing provide a solution to this problem.
List of References
Bothara, K., 2008. Inorganic Pharmaceutical Chemistry. Mumbai: Nirali Prakashan.
Hasan, A., 2008. Handbook of Blood Gas/Acid-Base Interpretation. London: Springer-Verlag.
Notes., n.d. Various current brands available and their theoretical advantages & disadvantages. Web.
Ricco, A., 1997. Chemical and biological sensors and analytical electrochemical methods. New Jersey: The Electrochemical Society, Inc.
Toffelli, J., 2001. Special topics in diagnostic testing: blood gases and electrolytes. United States of America: Library of Congress.
Taguchi, G., Chowdhury, S., and Taguchi, S., 2000. Robust engineering. New York: McGraw-Hill.
Diagrams
Fig. 1: Miniaturized polarographic electrode
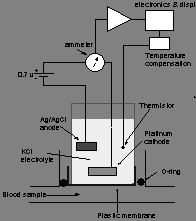
Fig. 2: Optical fluorescence microsensing