Introduction
Alkanes are the simplest forms of hydrocarbon and are composed of only single bonds between carbon atoms. The general structure of an alkane is CnH2n+2, where n is the number of carbons in the molecule. On the other hand, alkenes are hydrocarbons that contain at least one double bond between carbon atoms. The general structure of an alkene is CnH2n, where n is the number of carbons in the molecule. Various hydrocarbons, such as alkanes and alkenes, can be defined by different characteristics in addition to their diverse use amongst people.
The Structure and Characteristic Reactions of Alkanes and Alkenes
The Importance of Everyday Uses of Organic Compounds
Organic compounds are essential for the functioning of all living cells and play a vital role in many of the body’s processes, including metabolism, respiration, and reproduction. Organic compounds also help to regulate the Earth’s climate and are a major source of food and energy for plants and animals (Behmard, et al., 2019). Furthermore, humans have used organic compounds for centuries, from medicine to agriculture. Today, they are an essential part of people’s economy and their way of life.
The Environmental Impact of Global Hydrocarbon Consumption
The increased consumption of hydrocarbons such as natural gas and oil has led to increased levels of carbon dioxide and methane in the atmosphere. These greenhouse gases trap heat from the sun, causing the Earth’s temperature to rise. Global warming has led to several consequences, including more extreme weather events, rising sea levels, and melting glaciers (Wang, et al., 2018). The impact of global hydrocarbon consumption on the environment is thus twofold. Firstly, there is the direct impact of the increased greenhouse gases in the atmosphere, causing the Earth to warm. Second, the indirect impacts of climate change include more thrilling climatic events.
A Structural Formula for Any Named Alkane of at Least 4 Carbons
An example of an alkane having 4 carbon atoms is Butane, which has isomers n-butane and isobutane. Butane, with the formula C4H10, is an organic chemical, and it is a 4-carbon saturated hydrocarbon with a linear polymer structure (Behmard, et al., 2019). Hence, it is generally utilized in gasoline mixtures, alone or in conjunction with propane. It is also applied as a feedstock for the manufacture of ethylene and butadiene (Wang, et al., 2018). Butane, like propane, is extracted from natural gas or petroleum refining, and the two gases are commonly seen in combination.
Chain Isomerism and Structure’s Diagram of Isomeric Alkanes (Appendix A)
Chain isomerism is a structural isomerism in which the isomers differ in the order of their atoms. The simplest example is the alkanes, in which the isomers differ in the order of their carbons (Zhou, et al., 2022). The simplest alkane is methane (CH4), which has one carbon followed by ethane (C2H6), two carbons. The third one is propane (C3H8), with three carbons, and then Butane (C4H10), having four carbons. The fifth alkane is pentane (C5H12), with five carbons; the sixth is hexane (C6H14), with six carbons; the Seventh is heptane (C7H16), seven carbons and the eighth is octane (C8H18), with eight carbons. Additionally, the ninth alkane is nonane (C9H20), which has nine carbons, and the tenth is decane (C10H22), with ten carbons.
The Significance of the Term Homologous Series
A homologous series is a group of compounds with a similar chemical structure, where each molecule differs from the next by a repeating unit. Compounds in a homologous series frequently share a fixed set of functional units, resulting in equivalent physical and chemical features (Zhou, et al., 2022). The term is used to describe both organic and inorganic molecules. The concept is important in organic chemistry, as it predicts the properties of new members of the series based on the known properties of existing members.
Explanation and Equation Reactions of Alkanes – Combustion, Chlorination, Cracking (Appendix B)
Alkanes are relatively unreactive due to the stability of the carbon-carbon bonds. However, they will burn in oxygen to form carbon dioxide and water (Sankaralingam, Balamurugan, and Palaniandavar, 2020). The combustion reaction of alkanes can be represented in: (Appendix B: 1). Furthermore, alkanes can also be chlorinated, meaning that chlorine atoms can be added to the molecules (Boyadjian and Lefferts, 2018). That is done by reacting the alkane with chlorine gas. The chlorine gas reacts with one of the hydrogens on the alkane to form Hydrochloric acid (HCl), and that HCl molecule then reacts with another hydrogen on the alkane to form another HCl molecule (Zhang, et al., 2020). This process continues until all of the hydrogens on the alkane have been converted to HCl. Therefore, the reaction can be exemplified as: (Appendix B: 2). Alkanes can similarly be cracked, meaning that they can be broken down into smaller molecules. That is done by heating the alkane in the presence of a catalyst, such as iron (Sankaralingam, Balamurugan, and Palaniandavar, 2020). The heat causes the carbon-carbon bonds to break, forming smaller molecules. The overall reaction can be represented in: (Appendix B: 3)
Reasons why Alkanes are Relatively Unreactive
Alkanes are relatively unreactive because they have strong bonds between their atoms. Alkanes have strong single carbon-carbon bonds as well as strong carbon-hydrogen bonds. Since the carbon-hydrogen bonds are very weakly polar, no parts of the molecules with a large negative or positive charge might attract other molecules or ions (Boyadjian and Lefferts, 2018). The bonds in alkanes are formed by electrons being shared between atoms. The sharing makes the bonds between atoms very strong; breaking them takes a lot of energy.
Structural Formulas for Named Alkene of at Least 4 Carbons, Including Geometrical Isomers
The structural formula for an alkene of at least 4 carbons can be represented as follows: R-C≡C-R’, where R and R’ are each a group of one or more atoms bonded to the central carbon atom. A double bond links the central carbon atoms, and each of the R groups is bonded to one of the carbons in the double bond (McFarland, 2021). The R groups can be arranged around the central carbon atoms, resulting in different geometrical isomers of the alkene (McFarland, 2021). For example, if the R groups are arranged on the same side of the central carbon atoms, the alkene is said to be a ‘cis’ alkene. If the R groups are arranged on opposite sides of the central carbon atoms, the alkene is said to be a trans-alkene. Some examples include:
- 4-methyl-2-pentene.
- (CH3)2C=CHCH2CH3.
- 2-methyl-2-butene.
- CH3CH=C(CH3)2.
- 3-methyl-1-butene.
The Nature of the Double Bond
A double bond is a chemical bond in which two atoms share two electron pairs. Instead of the customary two bonding electrons involved in a single connection, this type of bond comprises four bonding electrons between atoms (Martins, et al., 2019). Double bonds are reactive due to the huge amount of electrons. The electrons are shared in pairs between the atoms, and each pair of electrons is known as a bond. Double bonds are stronger than single bonds, often found in molecules essential to life, such as DNA and enzymes.
The Difference in Reactivity Between an Alkane and Alkene
Alkanes and alkenes have extremely varied reactivity hence, alkanes infrequently react, but alkenes are quite reactive. Halides, alcohols, and hydrogen, to mention a few, rapidly react with alkenes (Martins, et al., 2019). One of the more well-known reactions is alkene bromination, the halogen addition reaction used to evaluate whether an organic compound is unsaturated or saturated. For example, when 2-hexene is combined with Br2, it becomes 2,3-bromohexane. Due to the removal of the double bond, the ending is returned to an alkane completion.
Reasons why Alkenes Undergo Addition Reactions
Addition reactions are one type of reaction that alkenes can undergo. In an addition reaction, a molecule (often H2 or Cl2) is added across the double bond. That results in the formation of a new single bond, and the double bond is broken. Therefore, alkenes undergo addition reactions because the double bond is electron-deficient (Kanan, et al., 2020). That makes the double bond a good target for attack by electron-rich molecules. One example of an addition reaction is the addition of H2 across a C=C double bond. That results in forming a C-H bond and the breakage of the C=C bond (Zhang, et al., 2020). The balanced equation for this reaction is: (Appendix C: 1).
How and why Bromine Water can be used to Test for an Alkene
Bromine water can be used to test for an alkene because bromine is a halogen. Halogens are elements that have a high affinity for electrons. When bromine is added to an alkene, the bromine takes the place of one of the hydrogen on the alkene molecule, creating a new molecule called a bromonium ion. The bromonium ion is unstable and will quickly decay, releasing bromine gas (Kanan, et al., 2020). The test is applied because an alkene can be distinguished from an alkane through a reaction with bromine water. Since bromine water is also a non-polar material, alkanes and alkenes are non-polar molecules that can dissolve in them.
The Importance of Polymerization Reactions of Alkenes
Polymerization is a type of chemical reaction in which simple molecules called monomers combine to form much larger molecules called polymers. The process of polymerization is extremely important in manufacturing many everyday alkenes products, including plastics, rubbers, and textiles. Many of the properties of these materials are determined by how the polymer molecules are arranged (Hou, et al., 2021). For example, the strength of a plastic material depends on the type of polymerization reaction that was used to make it. That means the polymerization reactions are extremely versatile and can create materials with a wide range of properties.
Conclusion
The extent of unsaturation or the existence of single, double, or triple bonds distinguishes hydrocarbons. Saturated hydrocarbons are hydrocarbons with only single bonds in their structure. For instance, alkanes and cycloalkanes are two types of saturated hydrocarbons. The only distinction is that cycloalkanes are cyclic, whereas alkanes are open-chain molecules. There are also unsaturated hydrocarbons, which are hydrocarbons with at least one double or triple bond. Simple unsaturated hydrocarbons with a double bond are known as alkenes, while simple hydrocarbons with triple bonds are known as alkynes.
Reference List
Behmard A., et al. (2019). Desorption kinetics and binding energies of small hydrocarbons. The Astrophysical Journal, 875(1), p.73. Web.
Boyadjian, C. and Lefferts, L., (2018). Catalytic oxidative cracking of light alkanes to alkenes. European journal of inorganic chemistry, 2018(19), pp.1956-1968. Web.
Hou, X. et al. (2021). Role of normal/cyclo-alkane in hydrocarbon pyrolysis process and product distribution. Journal of Analytical and Applied Pyrolysis, 156, p.105130. Web.
Kanan, S. et al. (2020). Recent advances in TiO2-based photocatalysts toward the degradation of pesticides and major organic pollutants from water bodies. Catalysis Reviews, 62(1), pp.1-65. Web.
Martins, F. et al. (2019). Analysis of fossil fuel energy consumption and environmental impacts in European countries. Energies, 12(6), p.964. Web.
McFarland, E., (2021). Unconventional chemistry for unconventional natural gas. Science, 338(6105), pp.340-342. Web.
Sankaralingam, M., Balamurugan, M. and Palaniandavar, M., (2020). Alkane and alkene oxidation reactions catalyzed by nickel (II) complexes: effect of ligand factors. Coordination Chemistry Reviews, 403, p.213085. Web.
Wang, Q. et al. (2018). Living polymerization of conjugated polar alkenes catalyzed by N-heterocyclic olefin-based frustrated Lewis pairs. ACS Catalysis, 8(4), pp.3571-3578. Web.
Zhang, T. et al. (2020). Difference of oxidation mechanism between light C3–C4 alkane and alkene over mullite YMn2O5 oxides’ catalyst. ACS Catalysis, 10(13), pp.7269-7282. Web.
Zhou, C.W. et al. (2022). Combustion chemistry of alkenes and alkadienes.Progress in Energy and Combustion Science, 90, p.100983. Web.
Appendixes
Appendix A: Structures of isomeric alkanes
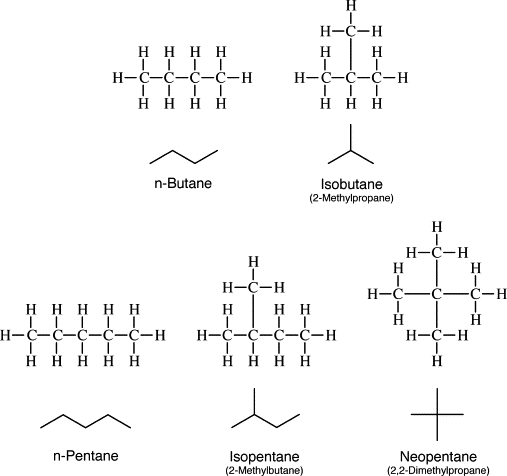
Appendix B: Equation Reactions of Alkanes
- CnH2n+2 + (n+1)O2 → nCO2 + (n+2)H2O
- CnH2n+2 + (n+1)Cl2 → nCH2Cl2 + (n+2)HCl
- CnH2n+2 → CnH2n + H2
Appendix C: Alkenes Addition Reactions
C2H4 + H2 → C2H6