Introduction
Alkenes are obtained through the electrophilic hydration process, where the hydrogen electrons are moved through heat from acids and alcohol. Double-bonded hydroxyl compounds are added to hydrogen for an electrophilic process (Wang et al., 2021). Electrophilic is a reverse process to dehydrate alcohols, resulting in alkenes, which use the reverse to make alcohol. The moving hydrogen protons are essential in the process due to the absence of electrons. This hydron breaks bonds and is vital in catalyst restoration. The mechanism mutually and practically makes reagents and fuels based on alcohol through a particular heat temperature. Z-3-methyl-2-pentene is dominant due to its substitution ability. 3-methyl 3-pentanol produces three alkenes, namely, 2-methyl 1-butene,z-3-methyl 2-pentene, and E-3-methyl 2-pentene.
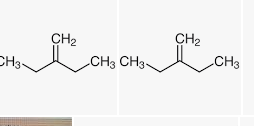
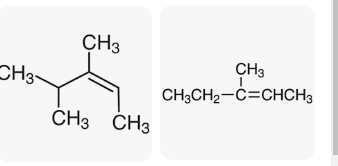
Alkene Ratio and Separation Principle
The carbocation is a rate-determining process with the tertiary alcohol dehydrating fast. HCl fumes in moisture; hence the liquified HCl is unsuitable for dehydration. Electron elimination in alkenes results in minor and significant bonds and products. It is, interestingly, a bond-breaking process that enforces the double bond into the essential elements in a ratio of 80:20, with the substituted being a very stable alkene (Wang et al., 2021). Sample solutions added to the gas stream separate various products in a column leaving pure gases through the carrier gases. The pressure gradient is applied through a permeable membrane to give the gas a driving force. Dense and porous membranes represent selective barriers in Chemistry in gas separation.
Alkene Yields
Theoretical yield balances the reaction into 1:1, where water is produced with cyclohexene as the main interest. Phosphoric in this reaction is a catalyst to achieve the result successfully. A particular amount of the alcohol needs to be changed into moles for easy calculations, for example, 4.10g cyclohexanol with 100.2g/mol molecular weight.
4.10 g cyclohexanol / 100.2 g/mol = 0.0410 mol (or 41.0 mmol)
Due to molecular balancing, the given moles of alcohol should produce a similar alkene in which the product’s weight is calculated afterward. Since the molecular weight of cyclohexene is 82.1g/mol, calculating the actual weight is easy using profound molecular equations.
0.0410mol x 82.1 g/mol = 3.36 g cyclohexene
Theoretical yield presents calculative with the best-given values of the chemicals used. Percentage calculation works with fractional values provided or the academic outcomes through a formula (actual/theoretical) x 100.
Column Length and Time Temperature Effect on Gas Separation
Longer columns enhance gas separation giving them enough space to evaporate the process efficiently. The temperature is essential in gas chromatography; an increase fastens the process, and cooling slows down (Wang et al., 2021). It affects the stationary phase and condensing time, allowing high amounts to evaporate and transfer very fast into the column, and lowering temperatures aid in resolving close peaks. Overlapping peaks are challenging through clustering that detects the availability of compound mixtures.
Different compounds take different times to elute at a constant temperature. Based on gas separation, some combinations may take longer, and identifying the boiling points eases the identification task. High boiling points take more extended periods to evaporate, and hence easy to differentiate the peaks and identify the height (Wang et al., 2021). Comparison to known compounds based on retention time through infrared and mass spectrometry is also applicable since all components are dissimilar.
Conclusion
Analytically, spiking evaluates between known product performance and a testing one. With a well-known mountain of the mix, it helps to detect the height of the unspiked combination. It identifies the sample matrix’s analytical conditions and recovery speed (Wang et al., 2021). Co-injection of 2-methyl 2-butene raises the peak of the mixture in the gas chromatography. Added substances in aliquots and performed calibration analyses of the version
Reference
Wang, H., Luo, D., Velasco, E., Yu, L., & Li, J. (2021). Separation of Alkane and Alkene by Metal-Organic Frameworks. Journal of Materials Chemistry A.