Benadryl is a white crystalline powder used as over the counter drug to treat allergies and hay fever among other ailments. Chemically, it is known as diphenhydramine hydrochloride (Garnett, 1986). Depending on the ailment, Benadryl which contains first-generation of antihistamines can be administered orally, intravenously, intramuscularly, topically or suppository. This organic compound was invented by George Rieveschl and has an IUPAC name 2-(diphenylmethoxy)-N,N-dimethylethanamine hydrochloride (Hevesi, 2007). The compound is known by such common names as Unisom, Diphenhist, Altaryl and Sominex among others. Diphenhydramine chloride has two functional groups: an ester and an acid. The organic compound has the following structure (Timberlake, 2009):
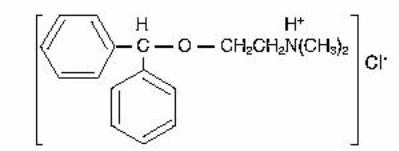
The compound is highly soluble in water and alcohol because it is a polar organic compound and has a molecular weight of 291.82. The solubility of the compound at 70.7° F equals to or greater than 100 mg/mL. However, the white crystalline powder is odorless. Diphenhydramine hydrochloride has a bitter numbing taste and a pH of 4 – 6 in 5% aqueous solution of the compound. Thus, the compound gives acidic solutions in water as well as neutralizing basic substances. Its functional groups enable it to react with both strong oxidizing and neutralizing agents. Due to this, the compound will gradually but slowly lose its white color and turn darkish when exposed to air. In addition, when mixed with organic compounds, the compound will react with them due to the functional groups. Moreover, the compound has a melting point of 331 – 338°F. Benadryl has a molecular formula comprising of seventeen carbons with two benzene rings (C17H21NO.HCl) (Garnett, 1986).
Diphenhydramine hydrochloride has a variety uses but is mainly used for treating allergies (Hevesi, 2007). Its use has stood the test of time since its invention in 1943 and approval by the United States Food and Drug Administration (FDA) back in 1946 as an antihistamine suitable for use by the public (Hevesi, 2007). Benadryl is the most effective drug for treating allergies. This compound reverses histamine build up in the body by blocking its synthesis by the body system. Natural, the body produces histamine. However, when not broken down or its synthesis, histamine accumulates in the body leading to allergic reactions manifested through skin rashes, and itching skin and throat. Hence, the drug will block histamine production and release it. It counters the effects of histamine by inhibiting smooth muscles’ response to histamine using its H1 receptor (Paton & Webster, 1985). It is also used to treating other symptoms of common cold throat such as coughing, watery eyes, runny nose and sneezing (Paton & Webster, 1985).
The drug is also used together other drugs to treat mild cases of muscle stiffness. This is usually the case in individuals suffering from mild cases of Parkinson disease and side effects of Meier-Gorlin syndrome also known as ear-patella-short-stature syndrome. This is a genetic psychiatric disorder which causes mild muscle stiffness and loss of control of body movements. These conditions are caused by overproduction of acetylcholine. Thus, Benadryl acts by blocking the receptors responsible for production of acetylcholine. Historical, this drug was used to treat insect bites, induce sleep and cause relaxation. However, the drug should not be used to induce sleep in children below the age of twelve unless on recommendation by a doctor. Neither should it be used by individuals with skin diseases and pregnant women (Simons, July 1990).
Some of the side effects of this drug include drowsiness, blurred vision, dryness of oral cavity and stomach upset which may lead to constipation. Moreover, some individuals may develop allergic reaction to this drug which may cause itchiness and skin rashes. Some may also suffer from difficulties in coordination of body movements. Generally, this drug is safe for use by individuals and its side effects are not life threatening in most cases. Interestingly, Benadryl is the first drug to be approved by PAD to be used as an antihistamine by the public in the United States. It has withstood the test of time to become the best histamine drug currently in the market.
References
Garnett, W. (1986). “Diphenhydramine”. Am Pharm (2): 35–40.
Hevesi, D. (2007). “George Rieveschl, 91, Allergy Reliever, Dies”. The New York Times. Web.
Paton, M., & Webster, D. (1985). “Clinical pharmacokinetics of H1-receptor antagonists (the antihistamines”. Clinical Pharmacokinetics 10 (6): 477–97.
Simons, K et al. (1990). “Diphenhydramine: pharmacokinetics and pharmacodynamics in elderly adults, young adults, and children”. Journal of Clinical Pharmacology 30 (7): 665–71.
Timberlake, K. (2009). General, Organic, and Biological Chemistry: Structures of Life. New York: Prentice Hall.