Introduction
Names of Reaction Components
The name of the substrate is Hydrogen Peroxide (H2O2), which will be targeted by the enzyme catalase found in the yeast solution, and the product will be water (H2O) and oxygen (O2). I will primarily detect the production of oxygen gas molecules (O2) to determine whether the reaction has occurred and whether the paper was lifted.
Chemical Relationships Between Components
The general relationship between enzyme activity or reaction rate and the amount of product detected is that higher enzyme activity results in a greater amount of product detected. However, if there is low enzyme activity, then fewer products will be detected.
Hypothesis and Predictions
Hypothesis
The activity of the enzyme catalase will change with exposure to different temperatures, showing an optimal temperature range for maximum enzymatic activity.
Prediction for Room Temperature
If my hypothesis is correct, then at room temperature, the enzyme activity will be moderate, and the filter paper squares will rise to the surface at a moderate rate.
Prediction for Ice Water Bath
If my hypothesis is correct, then at cold temperatures, the enzyme activity will be lower, and the filter paper squares will rise to the surface more slowly or not at all.
Prediction for 37°C Temperature
If my hypothesis is correct, then at 37°C, the enzyme activity will be higher, and the filter paper squares will rise to the surface more quickly than at room temperature.
Prediction for Hot Water Exposure
If my hypothesis is correct, then at hot water exposure, the enzyme activity might be reduced due to potential denaturation of the enzyme, and the filter paper squares will either ascend to the surface at a slower rate or fail to rise entirely.
Figure 1 below illustrates the graph of the temperature effects on the enzyme catalase.
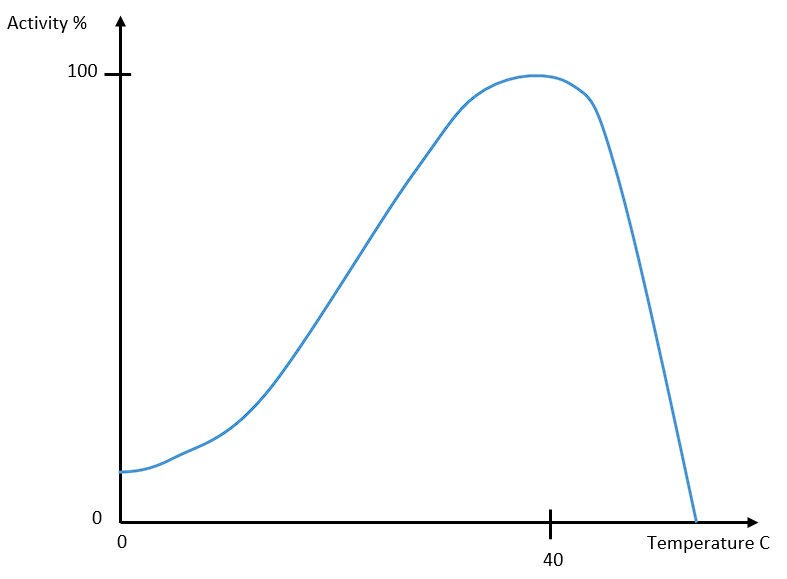
Figure 1
Conclusions
Controls
The water treatment in Test Tube #1 served as the control in this experiment. The specific purpose of the control was to establish a baseline for comparison and ensure that any observed activity was due to the presence of hydrogen peroxide and not some other factor.
Errors
Errors that might have occurred include inaccurate temperature control, inconsistent saturation of filter paper squares, or variations in the height of the H2O2 solution, which could have led to incorrect timing measurements and affected the overall results of the experiment.
Predictions
My predictions correctly assessed how each setup and corresponding reaction would unfold. Firstly, the control setup showed no activity because it lacked catalase to initiate enzymatic activity. Secondly, the room temperature setup demonstrated average catalase performance, with the filter paper rising moderately.
Thirdly, cold exposure slowed the reaction, resulting in delayed filter paper rising, as catalase was working at a lower rate. Fourthly, 37 °C demonstrated the best results since it is the most optimal temperature for catalase activity (Lundblad & Macdonald, 2018). Lastly, hot exposure denatured the enzyme, preventing the reaction.
Factors Affecting the Rate of Reaction of an Enzyme
Another factor, besides temperature, that could be tested is the pH level. To test this factor, I could create a range of buffered solutions with varying pH levels and expose the catalase to each buffered solution (Lundblad & Macdonald, 2018). Next, I could perform the same filter paper square experiment to observe the effect of different pH levels on catalase’s function.
Reference
Lundblad, R. L., & Macdonald, F. M. (2018). Handbook of biochemistry and molecular biology (5th ed.). CRC Press.