Background
Instrumental techniques in laboratory chemistry encompass various methods for separating mixtures based on the physical states of the components. One of these techniques is distillation, which can be generally understood as a process of liquid evaporation followed by the collection of condensates. Distillation is a fractional and straightforward process, depending on the number of thermal characteristics of the components.
Simple distillation is used when components with very different boiling points are present in a homogeneous mixture. If the boiling points of the two components are close, simple distillation is not applicable; instead, fractional distillation should be used. In this case, the general condensation process is almost identical: the mixture is heated in a specially prepared flask, which allows the sample with the lower boiling point to evaporate first. Such a component is collected by collecting the condensate, which separates the mixture into pure components. When fractional distillation is used, a rectification column is typically used instead of a more straightforward setup to separate more complex mixtures, as shown in Figure 1.
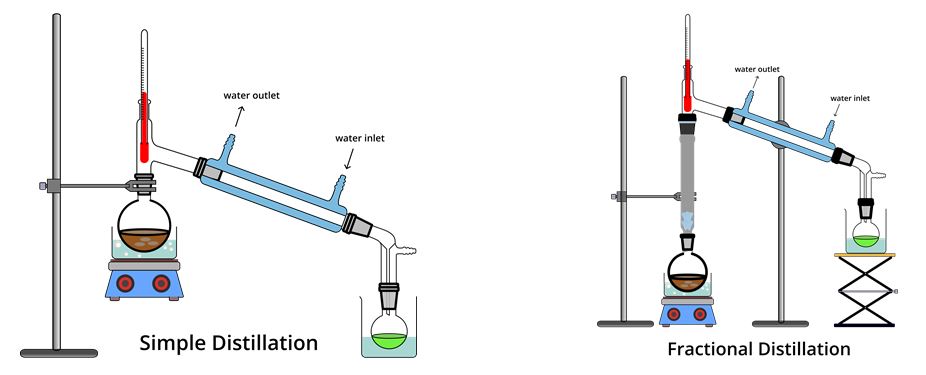
The experimental analysis involved measuring the temperature in a round-bottom flask or fractionation column using a thermometer, and the volume of condensate collected was placed in an Erlenmeyer flask. For both experiments, temperature and volume information were thus collected. The study of distillation curves enables the prediction of the number of components in a mixture based on the number of kinks in these curves. In particular, simple distillation is usually observed as a smooth curve without sharp steps or plateaus (Gilbert & Martin, 2010). In contrast, fractional distillation involves sharp temperature steps and plateaus, characterized by constant temperatures as the volatile component is removed.
Procedure
Simple Distillation
30 mL of ethyl acetate was placed in a 100-mL round-bottom flask with 2-3 balls of boiling stones and 2-3 drops of Alizarin Yellow R dye. The neck of the flask was sealed, and a sleeve was used to communicate with the 10-mL collecting flask, as shown in Figure 1 (left). A condensing phase was realized in the sleeve by a cold-water flow. The water flow was turned on, and the contents of the round-bottom flask began to be heated using an electric hotplate.
The heating was maintained at 300 °C, allowing roughly one drop of condensate to form and be collected in the flask every two seconds. At regular intervals, the temperature of the round-bottom flask was recorded with a thermometer, and the condensate was transferred into an Erlenmeyer flask. This procedure provided data on the mixture’s temperature and the volume of evaporated moisture.
Fractional Distillation
A 30 ml mixture of two organic components, ethyl acetate and n-butyl acetate, was used for fractional distillation. The mixture was placed in a 100-mL round-bottom flask with 2-3 boiling stones. After assembling the experimental setup shown in Figure 1 (right), a cooling water flow was started, and heating was turned on.
The heating was readjusted to ensure that approximately one drop of condensate formed and accumulated in the flask every two seconds, corresponding to a temperature of around 350–400 °C. A thermometer measured the temperature in the fractionation column at equal intervals, and the collected condensate was poured into the Erlenmeyer flask. Thus, the data collected included information about the temperature of the mixture and the corresponding volume of moisture evaporated.
Results
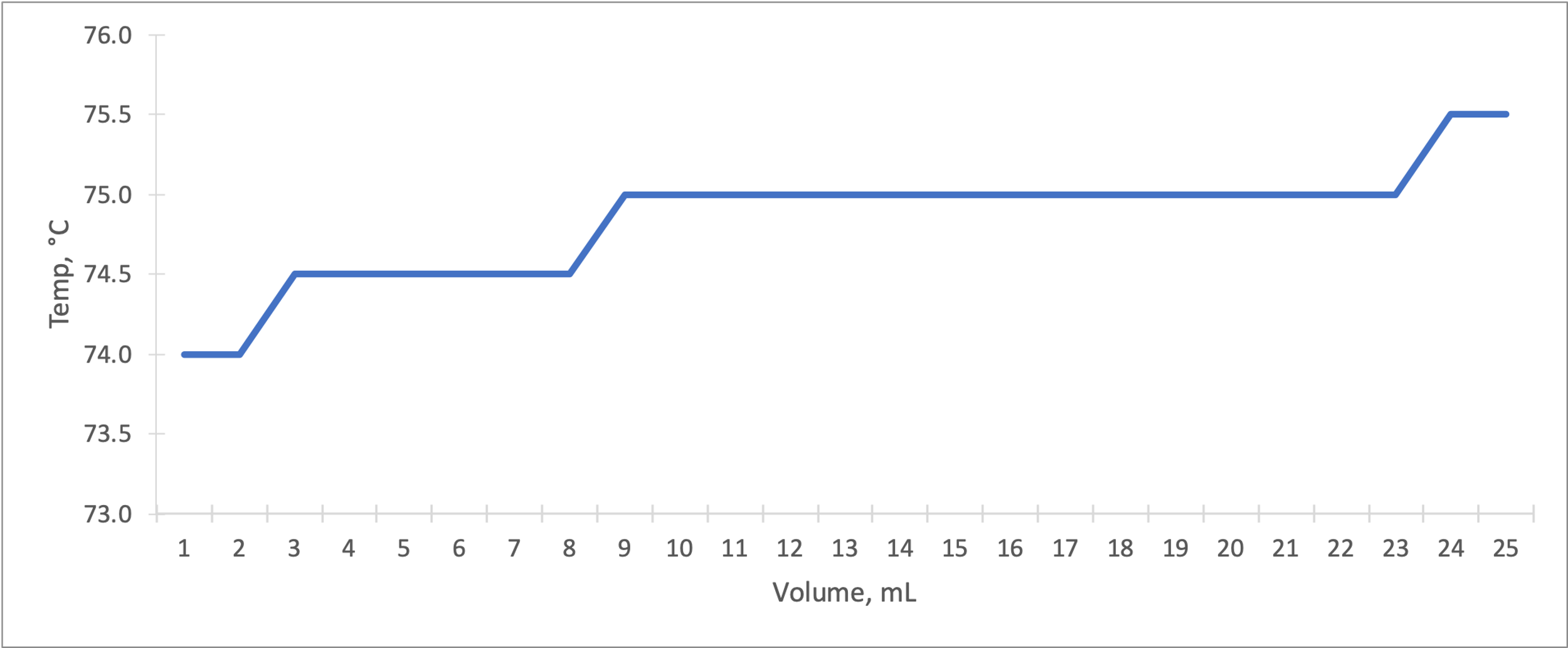
Temperature vs. total volume of distillate plots were obtained for both types of distillation. As shown in Figure 2, the simple distillation of ethyl acetate proceeded in steps, and after each temperature difference, it reached a plateau. Given the single-component composition of the mixture, the empirically determined boiling point of ethyl acetate in this experiment was 75-75.5 °C, and the subsequent plateau may correspond to water or dye distillation.
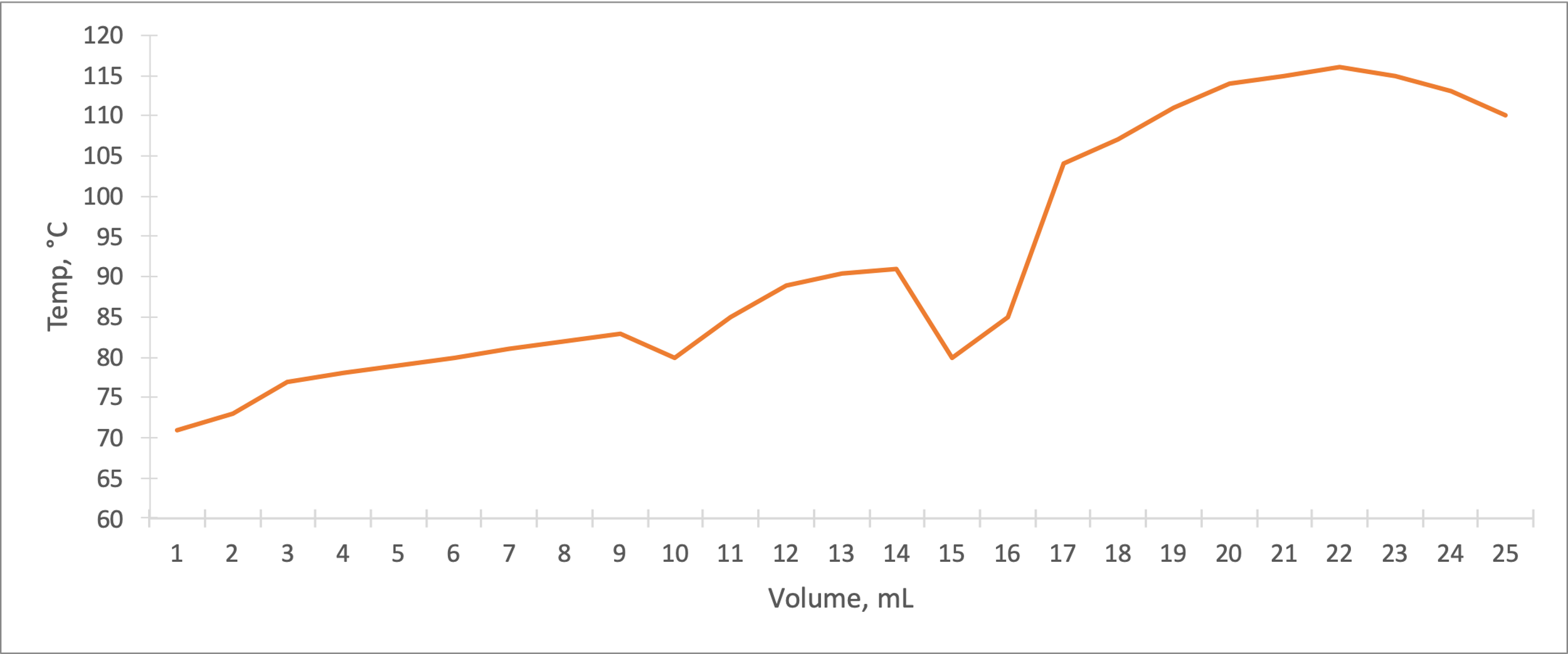
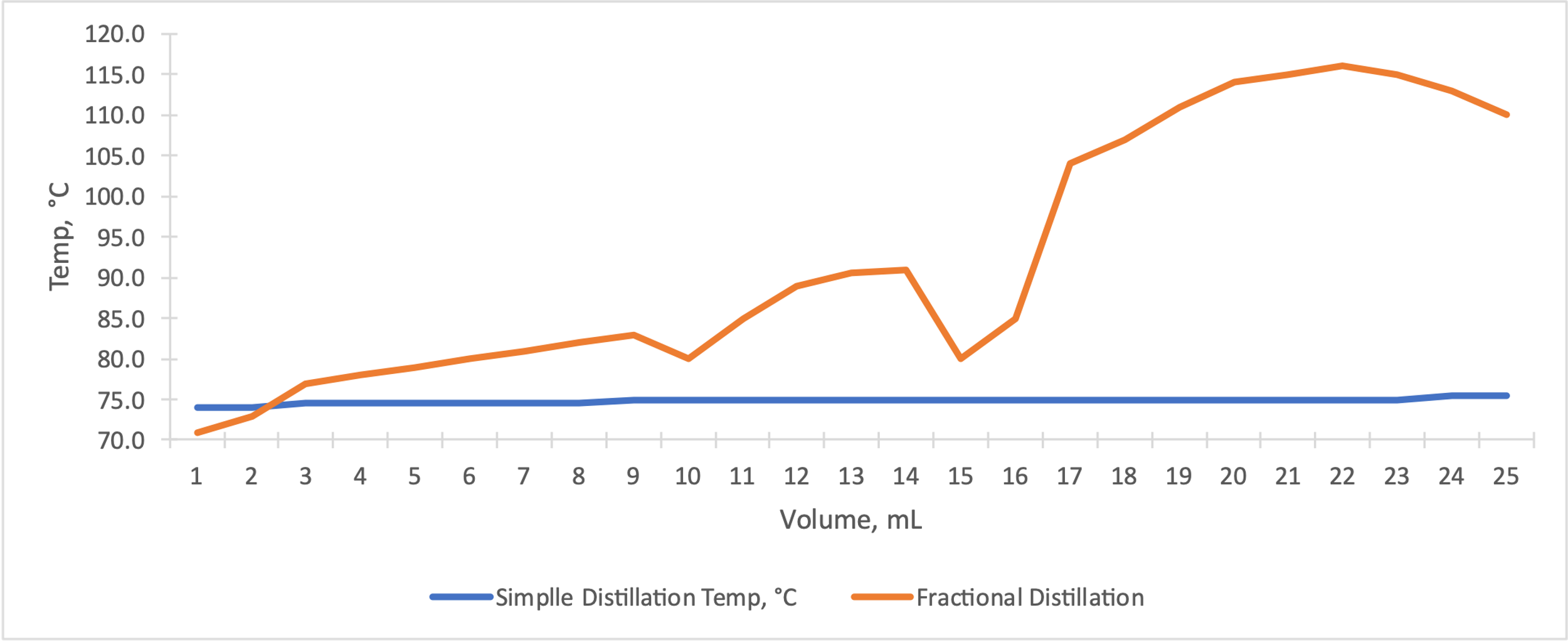
Figure 3 summarizes the fractional distillation of the mixture. The boiling point for ethyl acetate is between 77 and 80 °C, and for the second component, n-butyl acetate, between 110 and 116 °C. The differences between the graphs are palpable: simple distillation has more constant plateaus, in contrast to fractional distillation, in which the temperatures were rarely constant. In addition, the fractional distillation showed a decrease in temperature after the local maximum, indicating the complete removal of the more volatile component of the mixture. The differences between the plots seem more apparent when plotted on the same coordinate plane simultaneously, as shown in Figure 4.
Discussion
The graphs show the progress of the distillation steps, and in general, they can be described as successful, as they contain the basic patterns of these processes. The simple distillation contained more temperature plateaus than expected, which could indicate the presence of additional mixture components, including the dye. At the end of the simple distillation, the dye remained in the round-bottom flask because it was a less volatile component than ethyl acetate.
The fractional distillation contained three central plateaus, which could indicate the evaporation of each of the two components and water. For the mixture in the case of fractional distillation, the boiling points detected were 1-5 °C lower than predicted, which could indicate experimental errors. The higher boiling point of n-butyl acetate corresponds to a more complex organic structure than ethyl acetate.
The primary sources of errors in the experiment included imperfect plant assembly, reading errors, and analysis errors. First, it is possible that the setups were not completely sealed, which could have affected the quality of the temperature data. In addition, distillate collection could also have been performed with minor errors, which affected the final result. Second, reading the thermometer data under rush conditions could have needed to be more accurate. Finally, the interpretation of the graphs was relatively shallow, and it was for fractional distillation that the determination of the peaks could be inaccurate and required more detail.
References
Gilbert, J. C., & Martin, S. F. (2010). Experimental organic chemistry: A mini-scale and microscale approach. Cengage Learning.
Taleb, B. (2023). Simple vs. fractional distillation: A comparison. Psiberg. Web.