Introduction
Drug Classification
Pharmacology and medicine use the chemical properties of various natural and artificially created drugs for their treatment and development purposes. The focus of this report is the consideration of only the chemical properties of the drugs including metaproterenol sulfate, dyphylline, prednisolone, albuterol, salmeterol xinafoate, and theophylline. Metaproterenol sulfate is the generic name for corticosteroid MDI belonging to the class of rescue/reliever drugs. Dyphylline, also known as dihydroxypropyl theophylline, is rescue/reliever, it belongs to the class of “N-7 dihydroxypropyl derivative of theophylline” (Foye et al., 2007, p. 1245). Prednisolone belongs to the class of ∆-corticosteroids that display the features of anitrheumatic and antiallergenic agents and is 1-dehydro derivative of cortisone and hydrocortisone; it is rescue/reliever (Foye et al., 2007, p. 890). Next, albuterol is rescue/reliever and the drug belonging to the class of non-catechol selective β-agonists acting as the corticosteroid MDI and bronchodilator, while salmeterol xinafoate is the corticosteroid MDI and the long acting β-agonist belonging to the controller/preventer class of drugs. Finally, theophylline is also rescue/reliever drug belonging to the dimethylxanthine group (Foye et al., 2007, pp. 405; 406; 484).
Therapeutic Use
According to Foye et al. (2007) and Hernandez (2006), all the above mentioned drugs are used in pharmacology and therapy. Metaproterenol sulfate, dyphylline, prednisolone, albuterol, salmeterol xinafoate, and theophylline are bronchodilators serving as either rescue/relievers or controller/preventers in treating respiratory illnesses, and especially asthma (Foye et al., 2007, p. 1230; Hernandez, 2006, p. 205). The bulk of the drugs are administered via inhalation, while metaproterenol sulfate can also be administered orally in the form of tablets or pills.
Molecular Properties of Selected Drugs
Physical-Chemical Characteristics
Acid-Base Characteristics
The acid-base characteristics of the selected drugs demonstrate their composition of both strong and weak acids and only neutral bases. Metaproterenol sulfate consists of strong acids, H2SO4 and CH3, and neutral bases OH; dyphylline, albuterol, theophylline, and prednisolone consist of a weak acid CH2OH and similar OH bases. The composition of salmeterol xinafoate presents neutral bases OH only (Foye et al., 2007, p. 28; Hernandez, 2006, p. 37). Accordingly, the pKa values of the weak acids range from -2 to 12, while strong acids in the selected drugs have pKa values less than -2. In acid-base reactions, the pKa are the values that allow figuring out the pKb values, i. e. the base dissociation constant according to the formular ~14 – pKa. Thus, in metaproterenol sulfate, the pKa of H2SO4 is -3, so the pKb is ~14 – (-3) = 17. In prednisolone, the weak acid CH2OH has the pKa of 11.4, and the pKb is ~14 – 11.4 = 2.6.
Overall Polarity and Its Effect on Solvation (Hydration), Solubility, Dissolution, Partitioning between Phases
The selected drugs are characaterized by domination of polar molecules. Metaproterenol sulfate’s non-polar molecules include only carbon compounds like CH2 and CH3, while OH, HC, CH, HO, and H2SO4 are polar, which means that salvation, solubility, and dissolution are possible only in solvents of the same polarity, while partitioning coefficient is greater for metaproterenol sulfate with water. In dyphylline, only CH3 is non-polar, while CH2OH, OH, and H3C display polarity. Prednisolone is mostly non-polar as CH3 is in its contents, while polar molecules include only OH and HO ones. Albuterol is also characterized by domination of non-polar CH3 molecules. Only polar molecules OH, H, and N are observed in salmeterol xinafoate, while theophylline contains the bulk of polar molecules and a single CH3 non-polar molecule. These characteristics mean that drugs with dominant polar molecules have water-related salvation, solubility, and dissolution and the high partitioning coefficient, while predominantly non-polar drugs are dissolved in water-phobic substances with lower partitioning coefficients.
Similarities and Differences of Chemical Structures
The selected drugs display structures that differ by their acid-base features, polarity, and chemical geometry. The chemical structure of metaproterenol sulfate is (Flower, 2002, p. 114):
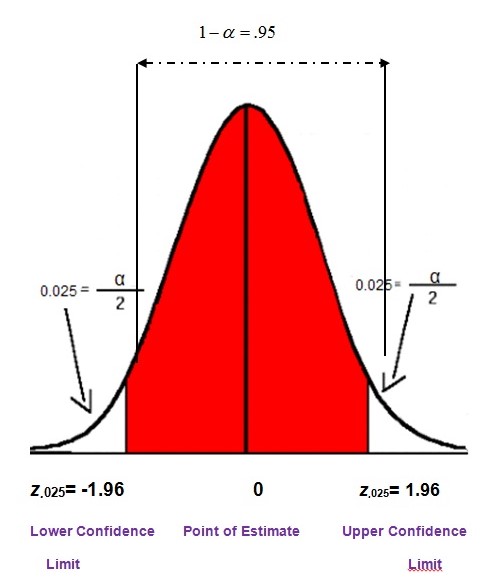
The chemical structure of dyphylline is (Foye et al., 2007, p. 1245):
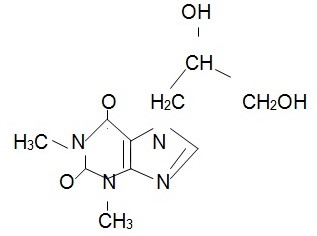
The chemical structure of prednisolone is (Foye et al., 2007, p. 888):
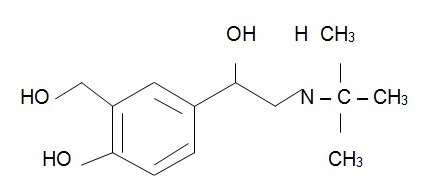
The chemical structure of albuterol is (Foye et al., 2007, p. 405):
The chemical structure of salmeterol xinafoate is (Foye et al., 2007, p. 35):
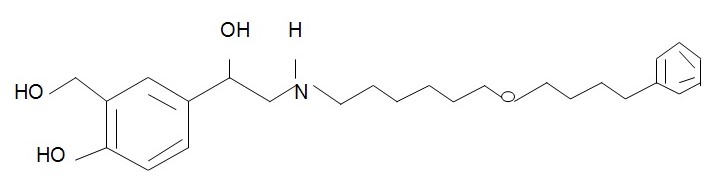
The chemical structure of theophylline is (Foye et al., 2007, p. 484):
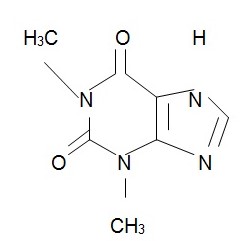
All the six drugs are characterized by displaying strong acids and neutral bases in their structures. The polarity is dominant in four out of six drugs, and only prednisolone and albuterol have non-polar molecules either dominant or exclusive in their structures. The geometry of the structures selected includes three linear structures (albuterol, prednisolone, and salmeterol xinafoate), a triginal planar (dyphylline), a trigonal bipyramidal (metaproterenol sulfate), and a T-shaped structure of theophylline.
The Possibility of Intra and Intermolecular Drug Binding Interactions
Covalent Bonding
The above discussed structures reveal opportunities for intra and intermolecular binding interactions on the whole and covalent bonding in particular. Thus, metaproterenol sulfate has a double bond between CH and two CH3 molecules. In dyphylline, there is a covalent binding between CH and the molecules of H2C and CH2OH. Albuterol displays a quadruple binding of carbon with three CH3 molecules and nitrogen. In theophylline the covalent bonding of nitrogen with H3C and O can be observed. Finally, only prednisolone and salmeterol xinafoate display no actual or potential covalent bindings because of their mainly linear and non-polar structures.
Electrostatic Forces
The concept of electrostatic force is hardly applicable to the selected drugs, as well as to the majority of drugs on the whole, because such a necessary condition for electrostatic bond, or ionic bond as it is also known, to form is the interaction between the two differently charged ions, one of which is the ion of a metal (Flower, 2002, p. 154). The selected six drugs do not possess any metals in their molecular structures, but mainly gases and acids, and therefore no electrostatic processes are actually possible within these drugs or between them unless any metal molecules are added to their intermolecular interactions’ process.
Stereochemical (Structural) Features
Optical Isomerism
The selected drugs also display considerable similarities in regard to their structural features and especially the stereochemical ones that illustrate the location of molecules within the structure of every particular drug. The notion of optical isomerism is applicable to the selected six drugs as the process when the similar or close chemical elements are arranged in a different way in one molecule that they are arranged in another one (Hernandez, 2006, p. 65). Thus, N, O, H, and C are obvious optical isomerism examples as they are present in all drugs’ structures but their arrangement is different from structure to structure.
Geometric & Conformational Isomerism
The presence of geometric isomerisms in the structures of the selected drugs is also obvious but no so common to all the structures and optical isomerisms are. Thus, the geometric isomerism can be observed between metaproterenol sulfate, albuterol, and salmeterol xinafoate in the symmetrical location of the double H3C/CH3 molecules. The conformational isomerisms are observed in metaproterenol sulfate, dyphylline, prednisolone, albuterol, and theophylline. Only salmeterol xinafoate displays no sings of conformational isomerism in its molecular structure.
Effect of Molecular Properties on Biopharmaceutical Features of Drugs Affecting
Drug Release, Absorption, Transport & Distribution
The above discussion reveals that molecular properties of the selected drugs affect their biopharmaceutical features in various ways. After the administration, the drug is transported by blood to the center of activity, i. e. the area of the organism in need of drug treatment. The process of metabolism transforms the drug into the necessary elements, and then the drug is distributed in the organism. After the treatment the drug or its metabolite is excreted from the organism, usually with the help of kidneys (Kadam, 2008, p. 187). As the majority of the selected drugs have high polarity, their salvation is possible only in combination with the elements of the same polarity. Accordingly, administration, absorption, and transportation of drugs depend on the compatibility of the organism with the type of the drugs discussed.
Drug-Receptor Interaction
The selected drugs, as well as the bulk of other drugs, are aimed at causing an effect on the organism, and therefore they are bound to interact with the receptors located in the areas selected drugs are used to treat. According to Foye et al. (2007), the process of drug-receptor interaction is the basic form to monitor the organism’s response to the administration of the drug, while the presence of hydrogen bonds in the drug’s structure provides for proper drug-receptor interaction (p. 484). All selected drugs display the wide variety of such bonds and hydrogen is one of their basic constructive elements, which means that these drugs are to cause proper drug-receptor interaction.
Biotransformation Pathways by Considering the Effect of Physical-Chemical Characteristics and Structural Features of Drug Molecules
There are two basic pathways of biotransformation of drugs, which takes place in the liver, including mineralization, i. e. the process resulting in formation of CO2, H2O, and NH3, and metabolism that usually results in metabolizing and excreting all other elements from the organism with the help of salvation (Hernandez, 2006, p. 291; Kadam, 2008, p. 187). The selected drugs have specific biotransformation pathways consisting of two main stages, phase I and II, under which the metabolites are first formed through the use of oxidative and hydrolytic reactions, and then are transformed into water-soluble compound elements with the help of enzymes and the process of conjugation reaction. Given the high partitioning coefficient in the bulk of the selected drugs and their predominantly high polarity, the above processes facilitate their fast and completely excretion from the organism.
Factors Influencing Drug Metabolism
The factors that influence the process of metabolism of the selected drugs are numerous and range from the race, sex, and age of the person administering the drugs to the possible pathologies of this person and his or her overall health conditions, food habits, and interaction of the administered drugs with the other drugs potentially administered simultaneously and the overall organism peculiarities. Thus, Inuit race is the most adjusted to metabolism of drugs, while African nations are the less adjusted for this (Kadam, 2008, pp. 129 – 130). Aged people, women, and children also might experience metabolism problems. Finally, the drugs administered must be compatible with the organism’s individual peculiarities and other drugs administered simultaneously (Kadam, 2008, pp. 129 – 130). Accordingly, the factors influencing dug metabolism range from racial and national to such personal aspects as age, sex, drug compatibility and organism peculiarities.
Strategies to Manage Drug Metabolism
Simply defined, metabolism is “what an organism does to the drug” (Flower, 2002, p. 89), and proper metabolism is essential for the functioning of the whole organism and avoiding the damage that drugs not excreted properly might cause to it. Therefore, to manage metabolism of the selected drugs it is necessary to first determine the genetic peculiarities of the organism and see if the drugs are compatible with them. If yes, the drugs can be used in treatment under the professional supervision. If no, alternative treatment ways should be searched. Second, it is necessary to coordinate the drugs’ use with the administration of other drugs. In case of drug compatibility, again the supervised treatment is advisable; if the drugs turn out to be incompatible alternative drugs might be prescribed. Third, proper food should accompany the use of drugs for treatment as the elements obtained with food assist with metabolism of drugs. Finally, drug-receptor interaction should be monitored to trace metabolism and make any corrections.
Pharmacological Activity
Thus completing the report of the six drug types used to treat asthma and other serious respiratory diseases, it is necessary to note that metaproterenol sulfate, dyphylline, prednisolone, albuterol, salmeterol xinafoate, and theophylline are used as bronchodilators and function as either rescue/relievers or controller/preventers of the illnesses. The administration of the drugs is carried out through inhalation and orally (for metaproterenol sulfate only in the form of tablets), which helps at first hand to reduce the spasms and fits of asthma, ease the throat muscles, and let a human being breathe freely during the time of the drug effect and up to the next asthma fit.
References
Flower, D. (2002). Drug design: cutting edge approaches. Royal Society of Chemistry.
Foye, W. et al. (2007). Foye’s Principles of Medicinal Chemistry. Lippincott Williams & Wilkins, 6th Ediition.
Hernandez, M. (2006). Basic pharmacology: understanding drug actions and reactions. CRC Press.
Kadam, S. (2008). Principles of Medicinal Chemistry. Pragati Books Pvt. Ltd.