Abstract
The purpose of using stoichiometric relations in the study of chemical processes is to investigate the reactions taking place, determine the percent yield, and evaluate the observance of the law of conservation of mass. This work aimed to determine the feasibility of applying the law of conservation of mass in two chemical processes, namely, the interaction of sodium and potassium carbonates with excess hydrochloric acid.
The results showed that the yields of potassium and sodium chlorides were 94.7 and 91.8%, respectively, indicating high productivity of both processes. The non-ideality of the obtained yields indicated experimental errors made during the synthesis and measurements. Based on the data obtained, the results on compliance with the law of conservation of mass, and the assumptions of errors, the paper proposed options for experimental improvements, including spectrometric measurements to determine the purity of the substances.
Introduction
Stoichiometric processes in analytical chemistry refer to the study of numerical and quantitative relationships between reactants and products within a single chemical process. The study of stoichiometric relationships allows for a deeper investigation of specific interactions between substances and the prediction of product yields given input data. The advanced use of stoichiometric principles allows the purity of products to be determined by determining percent yields, assessing the quality of experiments performed, and identifying undesirable and intermediate processes within the equation.
In the present laboratory work, stoichiometric studies were carried out for two chemical processes, the reactions of the interaction of two alkali metal salts (potassium and sodium) with hydrochloric acid. The experiments aimed to investigate the practicalities of calculating the reaction equations and to study the percent yield for each process to achieve a more general objective, namely the verification of the law of conservation of mass in chemical reactions.
Data and Analysis
Two chemical processes were involved in the present work, the balanced equations shown in formulas [1]-[2]. The difference between both reactions was due to the nature of the alkali metal cation and the quantitative yields.
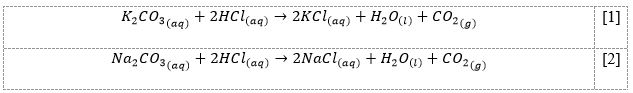
For calculations within stoichiometric processes, a mass of reactant from weighing results, molar amounts considering molar mass, coefficients in the processes, and a comparison of theoretical and empirical yields were used (LT, 2021; IET, 2023). Examples of calculations for the chemical process [1] are shown in Equations [3]-[7]. For the chemical process [2], the calculations were similar. The results of all measurements and calculations for both equations are shown in Table 1 and Table 2, respectively.
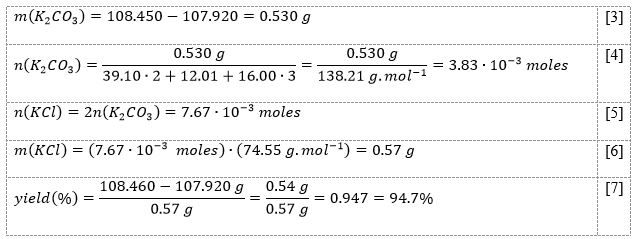
Table 1. Results of direct mass measurement of samples for the first and second reactions.
Table 2. Results of mass, theoretical, and percent yield calculations for the first and second reactions.
Discussion
The present experiment was based on stoichiometric calculations and aimed at studying the practical meaning of the law of conservation of mass. Two processes with salts of different alkali metals were carried out by interaction with excess hydrochloric acid. According to theoretical expectations, the yield of the products should have been 100%, provided that the law of conservation of mass was fully satisfied.
The measurements and subsequent calculations showed that the percentage yield of the reaction with potassium was equal to 94.7%, and for the reaction with sodium was slightly lower and amounted to 91.8%. In general, the results indicated sufficiently high product yields for both processes, indicating they were successful and free of impurity compositions. However, it is easy to see that the yield was not equal to 100% in both processes, which means that the empirically obtained product mass was lower than theoretically expected.
The results indicate a violation of the law of conservation of mass of substances in the composition of chemical processes: the mass of product yield is lower than the expected value of this indicator. The decrease in the percentage yield may indicate several errors in the experiment. First, it is not excluded that part of the product could have been lost during the transfer to the scales; the product could have stuck to the walls or the instrument, which lowered the final mass of chlorides (Nichols, 2022).
Secondly, not all the substances of the reactants quantitatively could have participated in the reaction, which could have been due to contamination of the substance or the presence of impurities. In other words, an increased mass of reactant with incomplete pure substance could have resulted in skewed mass measurement results and, consequently, a lower product yield. Third, it can be assumed that the conditions for the reaction were suboptimal, which affected the reactivity of the substances in the process (Nagwa, 2021). Thus, the law of conservation of mass is satisfied in both processes, but the associated experimental errors affected the lower percentage yield.
Conclusion
A study of stoichiometric ratios for chemical processes was conducted, allowing the law of conservation of mass to be evaluated by examining the percentage yield. The results showed that when potassium carbonate interacted with excess hydrochloric acid, 0.54 g (94.7%) of potassium chloride was obtained, and when sodium carbonate interacted in the same reaction, 0.45 g (91.8%) of sodium chloride was obtained.
The high percentage yields indicated the success of the reactions, while the lack of 100% yields in both cases indicated the presence of experimental errors. These included loss of substance, suboptimal experimental conditions, and contamination of reagents. In future studies, it is recommended that the ideal conditions for the experiments be more thoroughly investigated and that spectrometric measurements verify the purity of the reagents and products.
References
IET. (2023). How to calculate percent yield in chemistry. Web.
LT. (2021). Theoretical yield and percent yield. Web.
Nagwa. (2021). Question video: Identifying which factor does not affect percentage yield. Web.
Nichols, L. (2022). 3.4D: The unavoidable loss of recovery. Web.