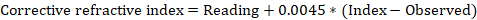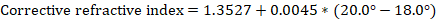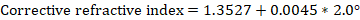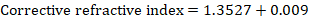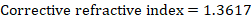Notebook Writeup
Purpose
The purpose of this lab is to perform a fractional distillation on the cyclohexane-toluene mixture created in the last simple distillation lab. In addition, the experiment determines the distillate’s components by refractive index.
Experimental Procedures
- The experiment was conducted similarly to Lab#2, Simple Distillation, with a few modifications.
- Fractional distillation equipment was prepared, and three test tubes were collected.
- A 30 ml round bottom flask was filled with a combination of Cyclohexane and Toluene at a ratio of 50:50, and three boiling stones were added.
- As a result of timely temperature regulation, distillation was successful.
- The first ten droplets were collected in the first test tube at 80℃.
- Each 2 ml increment in a graduated cylinder was analyzed for temperature and recorded.
- When the temperature reached 95 ℃, 10 drops were collected from the second test tube.
- As the temperature stabilized in the third test tube, 10 additional droplets were collected.
- Three test tubes were collected and taken to a refractometer for analysis.
Results
Fractional Distillation: BP of fractions #1 80 #2 95 #3 10
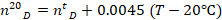
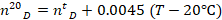
where T=19.9 °C
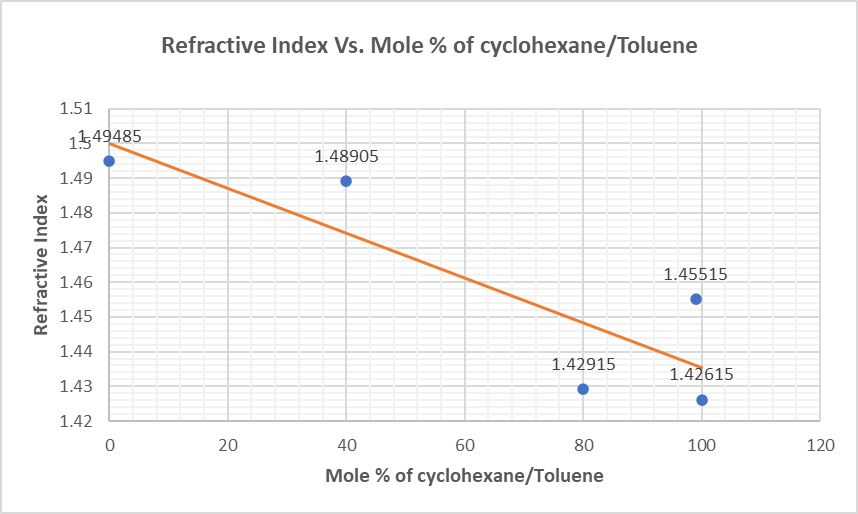
Summary
This experiment used fractional and simple distillation to isolate two chemicals from the mixture. This experiment found that fractional distillation is superior to simple distillation for removing two components from a mixture. Fractional distillation is more efficient than simple distillation, as depicted by the graph of temperature versus volume for each distillation curve. Also, it has been shown that repeated distillations of a solution improve its purity; hence the fractional distillation technique is preferable. Fractional distillation has the disadvantage of wasting material with each iteration of the distillation process. Some of it goes into the air, and some stay in the machine as condensation. While it is true that some amount of material will be lost during the experiment, regardless of our best efforts, this is an unavoidable loss and one that cannot be prevented.
Post-lab Questions
Question 1
When two liquids, such as alcohol and mercury, boil without mixing into a more complex substance, and there are high changes in temperature between their boiling points, simple distillation is used to separate the desired components. Distillation is the process of boiling a liquid mixture to separate its components. After reaching a certain height, the vapor is directed into the condenser’s inner chamber, where it is cooled by water. When the vapor cools, it becomes a liquid known as the distillate collected in a different container.
If the difference in boiling point is 25K or less, fractional distillation can be used to separate alcohol and mercury. Increasing the locations where the liquids can condense, a fractionating column boosts the efficiency of fractional distillation relative to simple distillation. In addition, glass beads or helices in a fractionating column give a huge surface area for the vapors to clash and lose energy, allowing for rapid condensation and distillation. In maximizing the effectiveness of fractional distillation, it is recommended to lengthen the fractionating column.
Question 2
- Boiling point: 80℃
- Composition: Toluene = 0% Cyclohexane = 100 %
- Composition of Toluene vapor
- Toluene = 20% Cyclohexane = 80 %
- When Toluene =20% and Cyclohexane =80%, the boiling point of L2 is around 75 degrees.
Question 3
In this case, the two factors include purity and solubility. The purity of a fraction in a fractional distillation is improved through an increase in the surface area of the fractional column. The longer it takes the vapors to ascend the column, the more efficient it is and the purer the fraction. Since the ability to dissolve into a uniform solution influences the efficiency of fractional distillation, solubility is another factor to consider.
Question 4
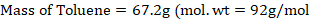
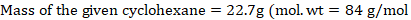
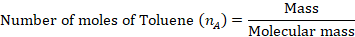
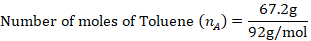
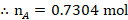
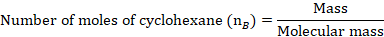
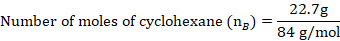
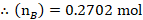
The mole fraction of Toluene
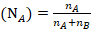
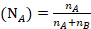
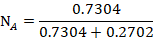
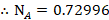
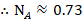
The mole fraction of Cyclohexane
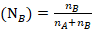
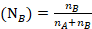
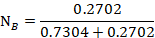
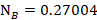
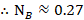
Question 5
- Partial vapor pressure of Toluene
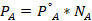
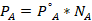
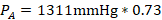
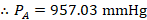
- Partial vapor pressure of Cyclohexane
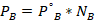
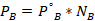
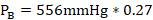
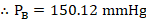
- Yes. The solution would boil at 100° because of an increase in the millimeters of mercury (mmHg)
- The composition of vapor of Cyclohexane Xc is given by
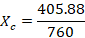
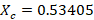
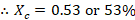
- The composition of vapor of Toluene Xt is given by
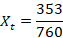
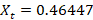
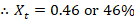
The composition of vapor of Cyclohexane Xc indeed follows the theory that Cyclohexane has a greater percentage than Toluene.
Question 6