The glucose molecule is a kind of carbohydrate described as a simple sugar. The chemical formula for Glucose is C6H12O6. It is present in honey and fruits and is the most abundant free sugar in higher animal blood. It is the energy source of cell activity. Hence it is critical to control its metabolism. Starch molecules, the plants’ primary energy carbohydrate reserve, are made up of many linear glucose residues.
The choice of Glucose in the study is essential since it helps the body to function correctly as the primary energy source in the body cells. Numerous health concerns can emerge when the concentration of Glucose in the blood is too low or too high. Most cells in the animal and human body use Glucose to produce energy, along with lipids and amino acids. However, it is the primary source of energy in the brain. In order to interpret information, chemical messengers and nerve cells in the brain require Glucose. The brain will be able to operate properly without it.
Everyday encounter with Glucose
Every cell in the human body needs the energy to carry out the metabolic operations that keep life going regularly. Glucose is the principal energy source for the muscles, brain, and bodily tissues. It also acts as a foundation for bigger structural molecules in the body, like glycolipids and glycoproteins (Elzagheid 2021). The human body strictly controls glucose levels because unusually low or high ones can lead to potentially fatal problems.
Chemical Formulae
Glucose has the chemical formula C6H12O6 or H-(C=O)-(CHOH)5-H. Its basic formula is CH2O, which shows that the molecule has two hydrogen atoms for every oxygen or carbon atom. Glucose is a form of sugar from the plant during photosynthesis and flows as a source of energy in human blood and other animals.
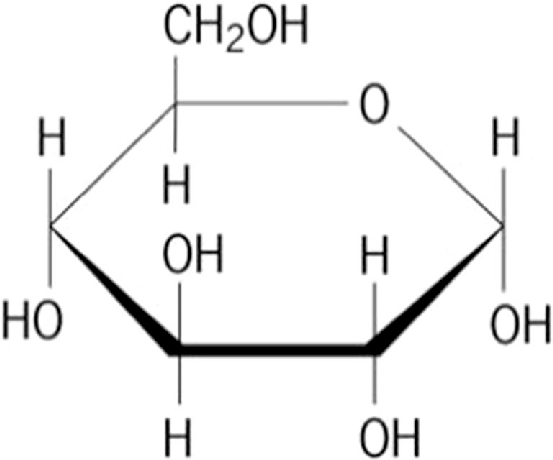
Properties of Glucose
Macroscale Observation of Glucose
The nanoswimmers are created by grafting polymer brushes on one edge of gold nanoparticles and then functionalizing the other end with glucose oxidase. In the concentration of Glucose, the resultant thermoplastic Janus gold nanoswimmers demonstrate effective acceleration with a frequency of approximately 120 body extents s-1. Comparing their kinematic performance demonstrates that the patched polymer bristles substantially increase the longitudinal dispersion of Janus gold nanoswimmers. The bacteria-mimicking Mobius gold nanoswimmers exhibit cooperatively chemotactic motility down a glucose concentration gradient that can be seen on the macroscale.
The quick and continuous amorphous-to-crystalline transitions of stage change substances like AgInSbTe and Ge2Sb2Te5 underpin phase change storage devices. Recognizing the crystallization procedure is critical because the crystallisation speed often restricts the maximal switching velocity of the devices. Although AgInSbTe and Ge2Sb2Te5 crystallize in significantly different ways from their melt-quenched forms, the nanostructural cause of the divergence is yet to be firmly proven. After heat processing, like furnace annealing, an arbitrary state has a variety of shapes and numbers of nanoscale atoms. The components of Glucose are hydrogen, oxygen and carbon. Glucose is often found in a cyclic structure in the solid state. Glucose has a circle of carbon atoms in this configuration. It is usually in a long chain shape when in liquid form.
New Science about Glucose
The proportion of haemoglobin A1c in patients is a test that shows lengthy blood glucose management. Once red cell hemolysates are electrophoresed, tinier peaks known as haemoglobin A1a, A1b, and A1c elute even before larger haemoglobin A rise. In multiple procedures, the “fast” haemoglobins are generated by the permanent binding of Glucose to haemoglobin. The proportion of glycosylated haemoglobin is determined by the median concentration of Glucose to which the red cell is subjected over time. Because red cells have an expected lifespan of 120 days, the proportion of glycosylated haemoglobin is an excellent indicator of blood sugar management during the previous weeks.
The new findings called into question the long-held linear form for sensing beta-cell Glucose. It recognized that the conventional model must be replaced by an oscillatory version (Banik 2020). It discovered how glucose metabolism is linked to insulin release in cells through the phosphoenolpyruvate loop. The research looked at medications that target the cycle, notably pyruvate kinase catalysts, that cause human beta cells to release more insulin while strengthening rather than weakening them. The team will optimise the lead molecules for upcoming phase 1 clinical studies.
Conclusion
Glucose is the most common form of sugar in the blood and the primary energy source for the body’s cells. Glucose originates from our meals, or the body might produce it from other chemicals. The circulation transports Glucose to the cells. Various hormones regulate blood glucose levels. Energy is liberated from Glucose during the respiration of cells and utilized to help create adenosine triphosphate. Plants employ photosynthesis to generate Glucose from water and carbon dioxide, and the Glucose is then utilized for the needs of the plant’s energy. Extra Glucose is frequently stored in the form of starch, which is degraded by other creatures that graze on plants.
References
- Banik BK. Green Approaches in Medicinal Chemistry for Sustainable Drug Design. Elsevier; 2020.
- Elzagheid M. Macromolecular Chemistry. Walter de Gruyter GmbH & Co KG; 2021.