Abstract
Titration can be used to quantitatively determine an unknown compound. In this experiment, a standardized sodium hydroxide solution was used to titrate an unknown weak acid and therefore determine the pKa and molar mass of that acid. The experiment was carried out according to laboratory manual number EQUL0857 and the data obtained was processed by drawing titration and first derivative graphs. The pKa values obtained were 2.55 and 2.59 while the molar mass values were 132.35g and 138.78g. The big difference between each pair of values was associated with experimental errors.
Introduction
One of the quantitative methods that can be used to identify an unknown compound (acid or base) is titration. Titration is the complete reaction of two solutions. When used to identify an unknown acid, it involves the reaction of the unknown weak acid solution with a known base solution. The known solution is known as the standard solution since its concentration is known and in titration terms, it is referred to as the ‘titrant’. During the titration, a specific amount of the unknown solution is reacted with the standard solution. This is done by slow addition of the standard solution into the unknown solution. To know the end-point of the reaction, an indicator or a pH meter is used. The end-point is technically known as the equivalence point and at this point, the masses of the unknown solution and the standard solution are the equivalent masses.
The aim of this experiment was to determine the pKa and molar mass values of an unknown weak acid through titration. The titrant used was a standard solution of sodium hydroxide. The hypothesis of the experiment was the acid was provided as a monoprotic acid.
Weak acids dissociate partially in water and at equilibrium, the dissociation is represented as follows:
HA (aq) + H2O(l) ↔ H3O+(aq) + A–(aq)
The equilibrium constant, Keq of the reaction is calculated as:
Keq = {[H3O+] [ A–] } / [HA]
For acids, Keq is represented as Ka and is referred to as dissociation constant. Since weak acids have very small Ka, pKa is used for convenience. During the reaction with a strong base, there is equilibrium alteration as the hydroxide ions react with the H3O+ ions. More acid dissociates so that a new equilibrium is obtained. At the equivalence point where the number of moles of acid is equal to the number of moles of base added, there is a sharp change in pH that can be detected by an indicator or a pH meter. The volume of the standard solution used up to this point is used to determine the unknown concentration.
Experimental Method
The acid sample size was determined according to the laboratory manual EQUL0857. After the sample size was determined, the pH probe was calibrated according to the calibration steps in laboratory manual number EQUL0857. To determine the pKa and the equivalent mass of the unknown acid, two determinations were carried out. The data obtained was treated by plotting a titration curve and a first derivative curve for each of the determinations. From the figures obtained from these curves, pKa and the equivalent mass for the unknown weak acid were calculated.
Results
Determination 1
Titration data.
Titration Curve
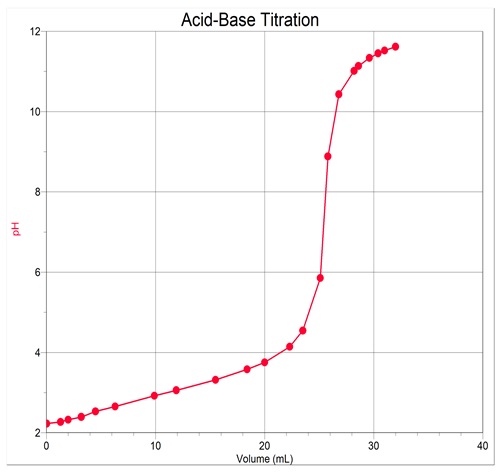
First derivative curve
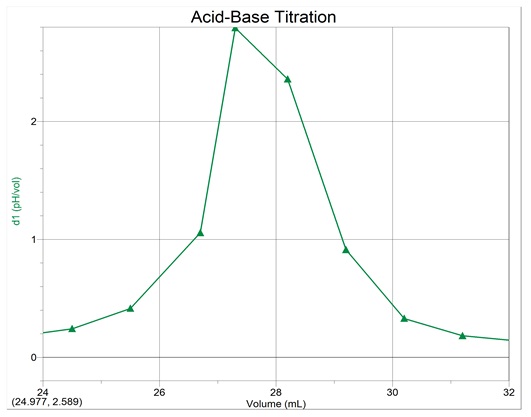
Equivalence-point volume = 27.3ml
Determination 2
Titration curve
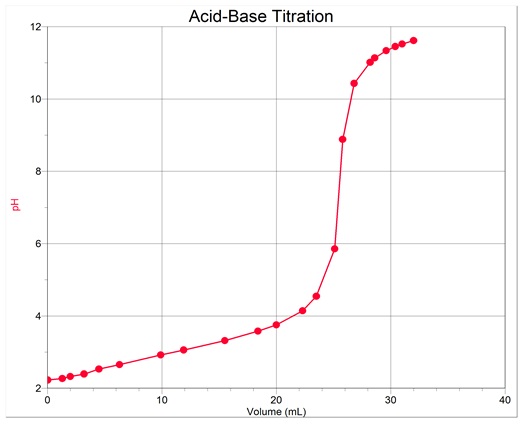
First derivative curve
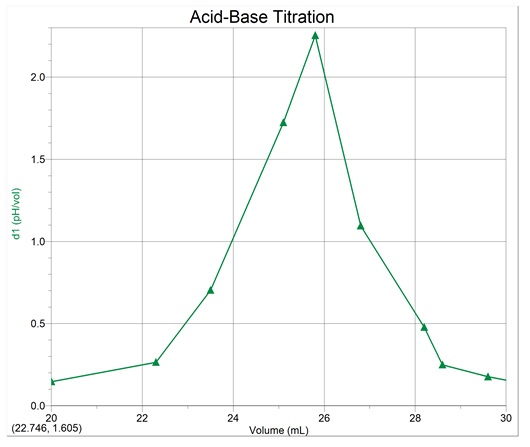
Equivalence-point volume = 25.8mL
Calculations
Sample size determination
Initial mass = 0.52g
Volume of NaOH used = 40.0 – 47.1 = 7.1mL
Mass needed for 25mL NaOH= 25*0.52 / 7.1 = 0.183g
Calculation of molar mass of the unknown acid
Molarity of NaOH = 0.05M
Volume of NaOH used = equivalence point volume – initial burrette reading
= 25.8 – 0.03 = 25.5mL
Moles of NaOH used = 25.5 * 0.05/ 1000 = 0.001275 moles
Mole ratio = 1:1
Moles of unknown acid used = 0.001275 moles
Mass of unknown acid used = 0.486 – 0.316 = 0.156g
Molar mass of unknow acid = 0.156g / 0.001275 = 122.35g
pKa Calculation for determination 1
pH = pKa + log10([A−]/[HA])
2.35 = pKa + log10(0.25/0.75)
pKa = 2.827
Average pKa = 2.59
pKa Calculation for determination 2
Average pKa = 2.55
Summary of results
The equivalence-point volume of NaOH for the reactions in the first and second determinations was 25.8mL and 27.3mL respectively. The average pKa obtained for determination 1 and determination 2 was 2.59 and 2.55 respectively while the molar mass was found to be 122.35g and 138.78g in determination 1 and determination 2 respectively.
Discussion
The titration curves for both of the determination experiments indicate that the unknown weak acid is a monoprotic acid. Monoprotic acids have only one displaceable H+ and therefore the whole titration curve represents one titration. Such a graph has only one end-point. Applying this finding, a mole ratio of 1:1 was therefore used in calculating the molar mass of the acid. In the determination of the pKa value of the acid, the absolute concentrations were not used but only the concentration ratios of [A–] to [HA]. The ratios were ¼, 2/4, and ¾ of the equivalence-point volume. The relation is true because at ¼ of the equivalence volume, ¼ of the acid has dissociated and ¾ remains in the initial protonated state. Three ratios were used in order to increase accuracy. The pKa values from the two determination experiments were within an acceptable range (2.55 and 2.59) but the molar masses had a large difference considering that they represent the same compound. The difference could be associated with experimental errors and approximations in reading the instruments. The sample sizes used also varied greatly from the calculated size. 0.170g and 0.156g were used instead of 0.183g.