Introduction and Aim
The FtsZ gene encodes for a protein that plays a role in mitochondrial cell division in bacteria such as E. coli (Birnboim & Doly 2001). The encoded protein, a GTPase, resembles eukaryotic tubulins in both function and structure. The aim of this practical was to isolate the FtsZ gene from a donor plasmid (pProEX) and ligate it into a second recipient plasmid, pBluescript KS II (pBKS II). This process, which involves excising a specific fragment of DNA (foreign genetic material) from a genome and ligating it into a plasmid, is called recombinant gene technology. It was first done in the mid-1970s. Common sources of template DNA for recombinant gene technology include cDNA and genomic DNA.
The practical proceeded in five sessions. The first session involved DNA manipulation, which involved two steps. The aim of the first step, small-scale plasmid isolation (‘Miniprep’), was to isolate a recipient plasmid, pBlue Script KS II (+), from an E. coli culture prepared the previous day. In the second exercise, a double restriction endonuclease digestion of the two plasmids, i.e., the donor (pProEX) and the recipient (pBKSII), was done using BamHI and HindIII. The aims of this step were to excise the gene of interest (Pc-FtsZ) from pProEX and prepare the recipient plasmid, pBKSII, for cloning. The two restriction endonucleases cleaved the DNA at specific sites to generate sticky ends that allowed the foreign fragment (Pc-FtsZ) to be inserted into the recipient plasmid.
Practical two involved the construction of a recombinant vector, pBKS II-ftsZ, using the restriction digests from the first lab session. Its aims were to compare the relative molecular sizes of the restriction digests with standards, confirm whether the restriction digestion was successful, and separate and purify the gene of interest for ligation. It entailed agarose gel electrophoresis to resolve the DNA into separate bands, excision to remove the desired fragments, and ligation of the gene into the pBKSII plasmid. The new pBKSII plasmid contained the Pc-ftsZ gene and could be inserted into E. coli in the next session.
The recombinant plasmids (containing Pc-ftsZ gene) from the above step were used to transform E. coli cells made competent through heat shock. The practical proceeded in two steps: (1) bacterial transformation (cloning) and (2) Southern blotting analysis of the cloned DNA. The objectives were to introduce ligated vector (pBKSII) into E. coli for cloning and analyse the cloned inserts using agarose gel electrophoresis and Southern blotting.
Since not all cells in the last session could take up the ligated vector, identifying transformed cells from the non-transformed ones was important. In the fourth practical session, an analysis of the Southern blot was done using specific probes. The main objective of this step was to confirm the presence of PC-FtsZ in the transformed bacteria. The practical also involved the screening of transformed bacteria using PCR to identify those carrying the pBKS II-Pc-ftsZ construct. The final (fifth) practical involved the screening of the Southern blot (gel) and testing the Taq polymerase colonies to confirm the results obtained in the previous session. Running a gel of the PCR colony screen obtained in the previous practical helped identify specific sequences in the transformed bacteria.
DNA fragments are separated based on size or molecular weight. Once placed in an electric field, fragments of small molecular weight move faster than large molecules (Asubel, Brent, Kingston, Moore, Seidman, Smith & Storuh 1995). This yields a distinctive banding pattern with each band representing a DNA fragment. The bands on agarose gels can be visualised through staining with DNA-staining dyes, such as ethidium bromide, followed by UV illumination (300-nm). In this practical, it was hypothesised that the band sizes will decrease from top to bottom of the gel because smaller fragments migrate further down the gel while large ones only move for short distances.
The blue/white screen is a method used to detect clones bearing a specific recombinant vector (a product of ligation) (Birnboim & Doly 2001). First, a gene of interest (Pc-ftsZ) is isolated from a donor cell and ligated into a vector (pBKS II). The recombinant vector is then used to transform competent recipient cells. The bacterial culture contains a substrate known as X-gal. Positive ‘tranformants’, i.e., bacteria transformed through successful ligation, will form white colonies while those that have not been transformed will remain blue. In this practical, since not all bacterial cells could be made competent through heat shock, it was expected that plating would give rise to both white and blue colonies. In this regard, only the E. coli cells from the white colony were selected for the PCR amplification step in practical four.
The Southern blot technique was used to confirm the presence of Pc-ftsZ gene in the constructed vector. The bound probes were detected using anti-DIG antibodies joined to alkaline phosphatase enzyme. Usually, a purple/brown colour indicates that the probes have hybridised to target DNA. Thus, purple/brown bands were expected where complementary probes were bound to the DNA bands.
Materials and Methods
Mini-Prep Step
This step entailed the extraction of plasmid (pBKS II) from E. coli. First, 1.5 ml of previously prepared bacterial culture was centrifuged at maximum speed for 1 min and the supernatant poured off to generate a dry pellet containing bacterial cells. This was followed by a re-suspension of the pellet in GTE buffer before swirling to generate a homogeneous mixture. A lysis buffer (1% SDS and 0.2 M NaoH) was added to the mixture to lyse the cells and release their contents. The high PH created was lowered using a neutralization buffer (3 M Potassium acetate). The mixture was then centrifuged at maximum speed for 10 minutes. Subsequently, 400 µl of the supernatant was transferred into another tube.
To precipitate the DNA, 1000µL of 100% ethanol was added to the contents of the tube. This was followed by centrifugation at top speed for 10 min to separate genomic DNA (the pellet) from DNA-binding proteins. The pellet was washed in 1ml and again in 500µl of 70% ethanol. This was followed by centrifugation at top speed first for 2 min and then for15 min. The pellet generated was dried using paper towels before being re-suspended in Tris-HCl buffer containing RNase A. The mixture was then incubated at 37°C for 10 min to denature RNA. The pellet was stored on ice for the next step.
Restriction Digestion
This step involved the use of restriction endonucleases to cleave pBKSII and bacterial plasmids at specific sequences. In this step, a mixture containing 3µL BamHI and HindIII, 11µL DH2O, and a buffer was added to two separate tubes holding 10µL and 15µL of pBKS II DNA and pProEX DNA respectively. The ttubes were then incubated for an hour at 37°C to facilitate digestion. Further digestion was stopped through incubation of the tubes at 65°C for 10 min.
Gel Purification
The products of restriction digestion were separated on 1.0% agarose gel that had been stained with µg/ml 0.5ethidium bromide. First, two samples consisting of the pBKS II plasmid digest and Pc-FtsZ insert were placed on the gel containing 5µl loading buffer. The nest step involved running the gel in a tank containing TAE buffer. A MW marker was loaded into the first and last wells on the gel. 20µl of each sample was loaded into different wells. Similarly, undigested samples were loaded into the gel to act as controls. The gel was run at 110V for 50 min to separate the fragments into distinct bands.
The bands corresponding to the plasmid and insert pattern were cut out before being transferred into a micro-centrifuge tube. Its mass was determined before adding QG buffer (three volumes). Subsequent steps involved repeated incubation, dissolution in isopropanol, and centrifugation of the samples at maximum speed to bind the samples to the spin column. The spin column was then washed in EB buffer to elute the DNA. The purified DNA was stored at -20°C for the next step.
Ligation
A mixture of pc-FtsZ, pBKSII, T4 DNA ligase, and a ligation buffer in specified volumes were added into a clean tube. The mixture was then incubated at room temperature for 20 min to allow ligation to occur. It was then stored at -20°C for the next step.
Heat Shock Transformation
Bacterial cells were made competent by first incubating them on ice for 20 min followed by heating to 42°C for 90 seconds. The competent cells were transferred to LB plates containing ampicillin, X-gal, and IPTG to screen for the blue/white colonies.
Southern Blot
In this step, four ‘6 x gel-loading’ samples containing circular pBKSII, dH2O, and DNA fragments (pBKS II and linear pBKS II) were prepared. The gels were labelled with DIG-markers and run at 120 V for 1 hour. Subsequently, the gels were subjected to denaturing conditions (1.5M NaCl and 0.5M NaOH) to degrade the DNA into single strands. After drying them, a piece of nylon membrane was aligned on each gel to transfer the DNA fragments (Southern blot). DIG-labelled Pc-ftsZ probes were used to hybridise cDNA on the membrane. The screening of the Southern blot involved an anti-DIG antibody to detect the DIG-labelled probes. A brown/purple colour indicated the presence of Pc-ftsZ gene on the blot.
Taq PCR Screening
The aim of this procedure was to identify transformed bacteria through PCR amplification of the pBKS II-Pc-ftsZ construct. Cells drawn from the white colony on the LB agar plates were placed in PCR tubes. PCR amplification was then done at different temperatures. The PCR products were separated on agarose gel to determine those bearing the gene of interest. Visualisation was done under UV light.
Results
In the first practical, the white pellet of plasmid DNA obtained was digested using restriction endonucleases to generate pBKS II and pProEX + Pc-FtsZ fragments. To separate these fragments for ligation, gel electrophoresis was done. The gel electrophoresis results are as shown in figure 1 below. The bands indicate fragments of different molecular sizes.

In practical three, the ligated DNA was used to transform competent E. coli cells. These cells were grown on LB agar plates containing X-gal and IPTG. Practical four involved the detection of transformed bacterial cells. The cells forming blue colonies in practical three were plated into fresh LB agar plate. The results indicated that out of the five colonies, four formed positive ‘transformants’ while one did not take up the recombinant vector.
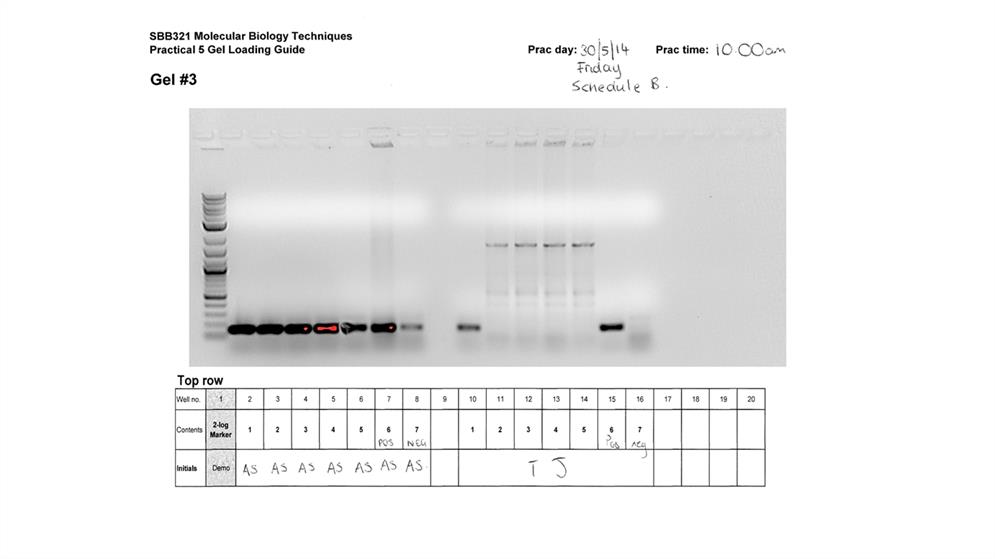
Practical five involved agarose gel electrophoresis to screen for the PCR results of the colony obtained above. Four different samples were loaded into the agar wells. These included (from left to right) pBKS II (undigested plasmid), linear pBKS II (restriction digestion products), ‘insert’ (Pc-ftsZ), and ‘ligation’. The sequence in which they were loaded on the gel is as shown in figure 2 below.
After running the gel at 120 V for 1 hour, the bands were blotted onto a nylon paper containing DIG-labelled probes before being viewed under UV light. The banding pattern generated in this practical is as shown in the figure below.
Discussion
Plasmids confer bacteria with certain essential qualities not encoded by the chromosomal DNA. Their sizes range between 1kbp and 200kbp. The aim of the practical was to isolate the plasmid DNA and use it as a vector for transferring a foreign gene (Pc-ftsZ) into E. coli. The mini-preparation step of isolating the plasmid DNA from E. coli cells involved four main procedures: (1) establishment of a bacterial culture, (2) lysis of E. coli to release cell contents, (3) removal of chromosomal DNA and proteins through precipitation, and (4) plasmid (pBlue Script KS II) DNA isolation. Chromosomal DNA and cellular proteins readily bind to one another to form precipitates (Wilson 1990). In contrast, plasmids, being smaller in size re-nature readily after SDS treatment and thus, come out as the supernatant. Thus, after this procedure, plasmid DNA can be recovered through ethanol or isopropanol treatment to precipitate them.
In this practical, restriction endonucleases, BamHI and HindIII, were used to digest pBlue Script KS II and pProEX plasmids. These enzymes cleave at specific sequences on the DNA molecule to generate sticky ends that facilitate the insertion of a foreign DNA fragment (Wilson 1990). The restriction products are separated on agarose gels, which resolves them based on their molecular sizes. Electrophoresis generates DNA bands of different sizes. In this practical, only those bands that had the right size were isolated and purified.
Figure 1indicates the banding pattern obtained when pBKS II plasmid (digested with HindIII and BamHI and treated with AP) in the first well followed by pProEX plasmid in wells two and three, as indicated by the ethidium bromide staining (red bands). Distinct bands can be seen in the wells containing products cut by the restriction endonucleases and treated with alkaline phosphatase (AP). AP treatment cleaves the phosphodiester linkage joining two sugar molecules that form the DNA backbone. This allows the endonucleases to cut at specific sequences.
In this practical, ligation of the DNA fragment (Pc-ftsZ gene) involved T4 DNA ligase, a bacteriphage that joins free DNA ends by filling the gap between the 3’ end and the 5’ termini with appropriate bases. It requires energy (ATP) and Mg2+ ions, which act as co-factors (Wilson 1990). After constructing the recombinant vector, the next step involved transforming the cells with this DNA. In this practical, the E. coli cells were first selected using ampicillin. Only cells that had the pBKS II plasmid could grow on a medium containing ampicillin. Moreover, LacZ gene, which encodes for β-galactosidase enzyme, allowed for blue/white screening. This enzyme breaks down X-gal (a substrate) into a blue-coloured compound. Thus, cells that contain this gene form blue colonies.
BamHI and HindIII cleave at sites near the lacZ gene. Thus, the insertion of the foreign gene (Pc-ftsZ) into this region will affect the functioning of lacZ gene, such that it will not be able to encode for a functional β-galactosidase protein. As a result, X-gal will not be metabolised into the blue-coloured compound. Thus, such a bacterial cell will form a white colony. In this practical, both white and blue colonies were obtained. To detect positive ‘transformants’ only E. coli cells obtained from the white colonies were selected. Heat shock treatment does not always make all cells competent to take up foreign DNA. This means that some cells will be transformed while others remain unchanged because they lack the ability to take up recombinant DNA molecules. In this practical, the cells were selected based on ampicillin resistance and blue/white screen.
Besides antibiotic and blue/white screening, PCR was used to screen the transformed bacteria, i.e., those that took up the pBKS II- Pc-ftsZ construct. Using two primers (T3 and T7) that are complementary to the vector sequences, the PCR step was done to amplify the foreign DNA. The PCR amplification involved entire bacterial colonies taken directly from the LB agar plate. The DNA from the colonies (template) was mixed with PCR reagents, including dNTPs, primers (T7 and T4), Taq polymerase, and a buffer. PCR involves a DNA polymerase activity that catalyses the replication of a target gene fragment. It proceeds in a chain of reactions that occur in alternating high and low temperatures. The initial temperature is often high to generate single strands from the double stranded DNA. A reduction in temperature allows the primers to bind to the template DNA at specific sequences. Replication of the DNA fragment requires elevated temperatures. In this practical, the PCR products were resolved on agarose gel.
Southern blotting was used to analyse the cloned inserts separated through agarose gel electrophoresis. The technique involved a capillary transfer of the DNA fragments contained in agarose gel to a nylon membrane followed by visualisation and detection of the fragments (Priefer 1994). In the practical, DIG-labelled probes that hybridised to the bands were blotted onto a nylon membrane. Probes only bind to complementary fragments on the nylon membrane. Thus, non-specifically bound probes could be washed off from the membrane using a different elution buffer.
In the practical, because the target fragments and the probes were complementary, washing off unbound probes required elevated temperatures and a strong detergent (SDS) concentration. The detection of the hybridised probes involved the use of anti-DIG antibodies joined to alkaline phosphatase enzyme (AP). AP removes a phosphate group from 5-bromo-4-chloro-3-indolyl-phosphate, which reduces nitroblue tetrazolium chloride into a purple precipitate (Wilson 1990). In this practical, the AS samples turned purple when treated with anti-DIG antibodies indicating the Pc-ftsZ gene hybridised successfully to the probes. The presence of this gene in the PCR products confirmed that the ‘foreign’ gene was inserted successfully into the pBKS II plasmid, which transformed the bacteria.
Practical Questions
Practical One: EX 1A
- Ampicillin was included in the growth medium (Luria Broth, LB) to select for antibiotic resistance. The LB medium contained all the nutrients necessary for bacterial growth. However, the inclusion of the antibiotic (ampicillin) ensured that only E. coli cells with plasmids (pBKS II) that confer them the resistance to ampicillin could grow. Thus, ampicillin resistance was used as a selectable marker in this experiment.
- ‘RNAse’ is included in the ‘miniprep’ step to degrade the RNA molecules in the mixture. Cell lysis releases cell contents, which include bacterial genome (chromosomal DNA and plasmids), RNA, proteins, and other cellular debris. This mixture is treated with ‘RNAse’ enzyme to remove RNA molecules.
- Plasmids can be purified from proteins and cellular debris through phenol/chloroform extraction. Ethanol or isopropranol is then added to precipitate the plasmid DNA (Wilson 1990). Affinity chromatography can also be used to bind the plasmid DNA and separate it from the protein/cellular debris mixture. The plasmids are released by altering the pH and salt concentration.
EX1 B
- A double (BamHI and EcoRI) digestion of the pBKS II plasmid without the insert (3.0 kb) will yield two fragments because there is a single BamHI site located in the EcoRI cleaving region. It is expected that the fragments will be 1400 bp and 1600 bp in size.
- Restriction enzymes have specific recognition sequences where they cleave. BamHI recognises TGG while EcoRI only cleaves the G (guanine) base in GGA. On the other hand, the recognition sequences for Kpnl and Sacl are CGG and GGA respectively.
Practical Two
- To visualise the resolved bands, ethidium bromide solution (0.5μg/ml) is added to the gel after electrophoresis. Caution is required when using this approach, as ethidium bromide is carcinogenic. The second approach involves adding the stain to the gel before casting it into slabs. However, this method lowers the migration of the DNA fragments and affects their ‘electrophoretic’ mobility (Brody & Kern 2004).
- A ‘submarine gel’ is completely covered by the buffer solution. Thus, loading of samples into the gel as well as electrophoresis occur under submerged conditions. ‘Submarine’ gels can allow an electric current to pass through because the buffer contains ions that carry electrons. Increasing the volume of the buffer prevents the gel from drying, which may affect electrophoretic separation. ‘Submarine gels’ facilitate sample loading and protect the gels from damage.
- Samples of undigested pBKSII and pProEX DNA were loaded into the wells on the agarose gel to act as controls. Undigested DNA formed a ‘smear’ while digested samples made distinct bands on the gel.
- Ligation involves the insertion of a ‘foreign’ gene fragment into the host cell DNA. For efficient ligation to occur, the fragment and the recipient DNA must have free 3’ and 5’ ends. Blunt ends lack free 3’ and 5’ terminals that are present in sticky ones.
Practical Three: Ex 3A
- The cells made competent through heat shock are incubated in LB medium to allow them to take up the plasmid DNA. Therefore, incubation facilitates transformation.
- Heat shock involves a drastic change of temperature from 0°C (ice) to 42°C within 90 seconds.
- Besides heat shock, bacteria can be made competent through chemical treatment. The E. coli cells are placed in a highly concentrated calcium chloride solution to make them competent.
- The plates are inverted before incubation to avoid other bacterial strains that may be present in the water condensing on the lid from contaminating the colonies growing on the LB medium.
- Ampicillin is added to the medium to screen for positive ‘transformants’, i.e. those that have the pBKS II- Pc-ftsZ construct.
- The addition of EDTA into the DNA strand terminates the synthesis of the probe. This happens because EDTA, a derivative of thymidine, lacks OH at the C-5 end.
- Besides Southern Blots, in-situ hybridisation and affinity chromatography can be used to detect the DNA fragments in the transformed cells.
- In-situ hybridisation and affinity chromatography are inexpensive and safer compared to Southern blots because they do not involve the construction of probes and primers. However, the strong solutions required to elute the DNA may affect its structure and integrity.
Practical 4A
- Besides capillary action, DNA can be transferred through vacuum blotting.
- The pre-hybridisation step helps to melt the DNA into single strands.
- A membrane can be re-probed if the time and incubation temperature did not allow optimal hybridisation.
Practical 4B
- The probe only binds to complimentary bases in the target sequence. Thus, it should be generated from cDNA, not the target DNA. In each PCR cycle, a temperature of 94°C allows ‘denaturation’ of the double stranded DNA to occur, 55°C facilitates the annealing of the primers, and 72°C helps in amplification.
- The blue colony is used as a control because it contains untransformed cells.
- The Pc-ftsZ gene can also be identified through microarray analysis of the colonies.
Practical Five
- Besides the use of radioactively-labelled probes, the DNA sequences can be detected using chemilumniscent dyes.
- As expected, a single band is visible in the Southern blot.
- Inserting the pBKS II-Pc-ftsZ construct into a suitable vector.
References
Asubel, F Brent, R Kingston, R Moore, D Seidman, J Smith, J & Storuh, L 1995, Current Protocols in Molecular Biology, John Wiley & Sons, New York.
Birnboim, H & Doly, J 2001, ‘A Rapid Alkaline Extraction Procedure for Screening Recombinant Plasmid DNA’, Nucleic Acids Research, vol. 7, no. 1, pp. 513-523.
Brody, J & Kern, S 2004, ‘History and Principles of Conductive Media for Standard DNA Electrophoresis’, Anal Biochemistry, vol. 333, no. 1, pp. 1-11.
Priefer, U 1994, ‘Characterization of plasmid DNA by agarose gel electrophoresis’, Advanced molecular genetics, vol. 8, no. 1, pp. 26-37.
Wilson, K 1990, Preparation of genomic DNA from bacteria: Current Protocols in Molecular Biology, Greene Publ. Assoc. and Wiley Interscience, New York.