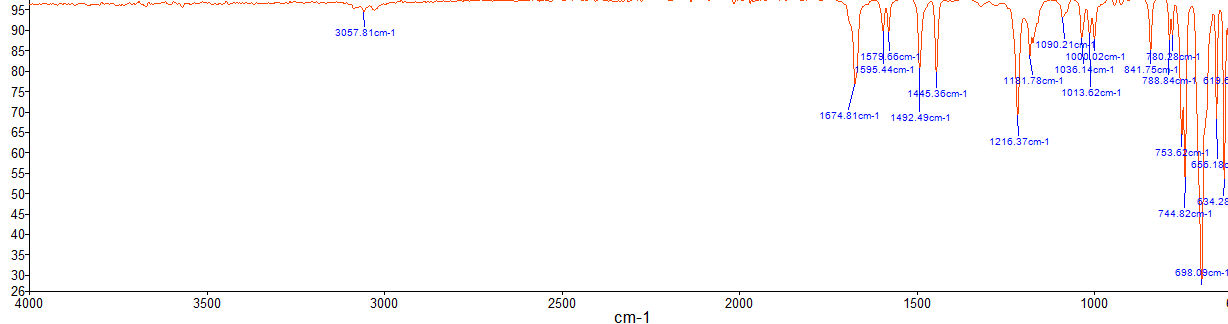Background
In laboratory work, Benzopinacol was transformed into Benzopinacolone in an acid-catalyzed dehydration reaction. This process is based on protonation of the diol followed by the removal of water and atomic rearrangement leading to the formation of a multiple bond within the molecule (Fig. 1). An acidic catalyst (for example, sulfuric acid) is added to the sample used, resulting in the protonation of the hydroxyl group. This makes the oxygen in the hydroxyl group more electrophilic and looser to detach from the carbon skeleton, leading to the formation of water as a byproduct of the reaction.
An additional reason for the detachment of the OH2+ group is the energy instability of the protonated alcohol, particularly the strong electrostatic repulsion between the oxygen atom and the electron-rich carbon atom. The intermediate carbocation formed during the detachment experiences electron density instability, and the excess electron density from the oxygen atom migrates to the neighboring carbon to stabilize it.
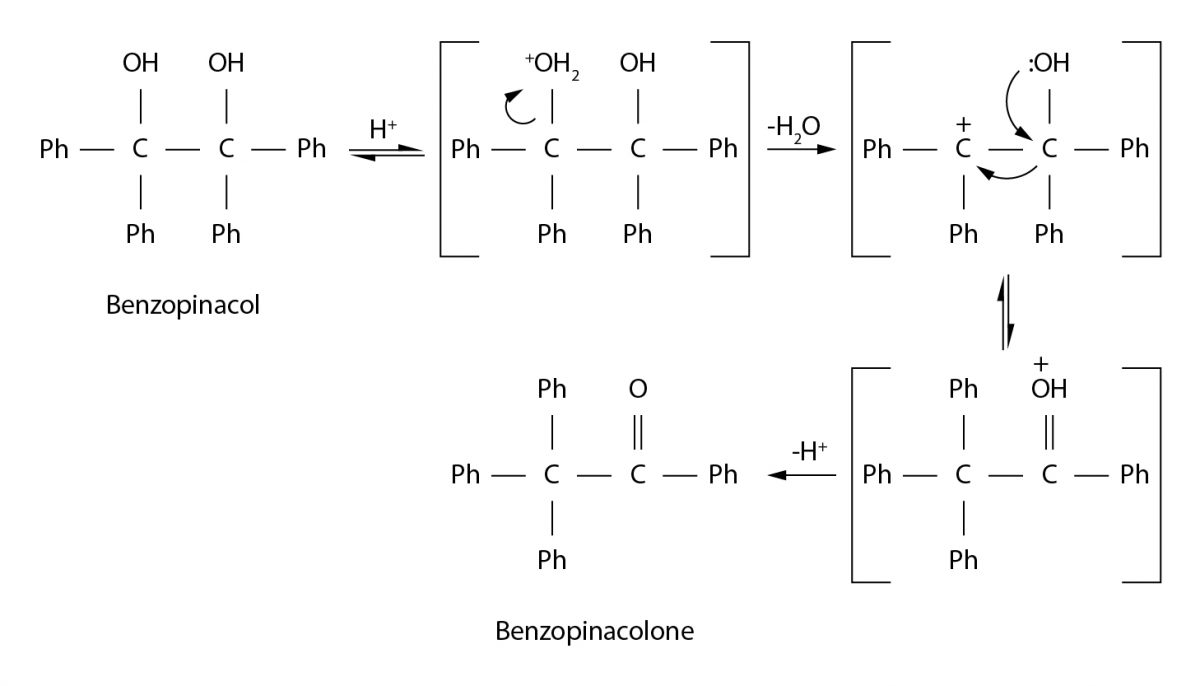
Due to density transfer to oxygen, the carbon becomes electron-oversaturated, and to minimize this instability, one of the phenyl groups is rearranged into a neighboring carbon. The secondary carbocation has increased stability, but the electron-deprived oxygen in the hydroxyl group acquires a positive charge. Deprotonation allows the stabilization of this carbocation, and this process most often occurs under the action of the added base, resulting in the formation of a stable ketone.
Experimental Procedures
50 mg of Benzopinacol was transferred to a reaction tube to which 0.25 mL of acetic acid and iodine were added and used as a catalyst. To dissolve the Benzopinacol crystals, the mixture was slowly heated in a water bath with hot sand, and after the solution had cooled, 1 mL of ethanol was added to the mixture to initiate the crystallization process. Vacuum filtration on a Hirsch funnel was then used to remove reaction residues and isolate the solid. The resulting sample was used to determine the boiling point and perform IR spectroscopy.
Table 1. Summary data for Benzopinacol (reagent) and Benzopinacolone (product)
Discussion
This work aimed to synthesize Benzopinacolone from Benzopinacol using acid-catalyzed dehydration. The molar ratio between the reagent and the product was 1 to 1, so the expected molar yield of Benzopinacolone was 0.0001364, equivalent to 0.052 grams of the substance (theoretical yield). The product yield was 70%, 25 percentage points lower than expected.
Some reasons for the lower yield include loss of substance, weighing errors, or unwanted extraneous processes if the reaction vessel was not sterile. The purity of the obtained substance can be verified by thermometric analysis: the measured boiling point was 181-182 °C, which is in complete agreement with the reference data (FS, n.d.). Additional verification can be done by infrared spectroscopy.
Table 2 shows the spectroscopic results for the solid residue after filtration, Benzopinacolone. It can be seen that no O-H vibrations were detected in the analysis of the product (Appendix A), and the other bonds were determined. This confirms that the resulting substance lost its hydroxyl group but acquired the carbonyl group shown at 1674 (C=O). In other words, IR spectroscopy also confirms the purity of the obtained substance. To summarize, both instrumental analysis methods showed that Benzopinacolone was pure and free of impurities, which means that instrumental or random errors could explain the moderately high percentage yield.
Table 2. Characteristic peaks for the IR spectrograms of the reference substance and product
Reference
FS. (n.d.). Benzopinacolone, 97%, thermo scientific. Fisher Scientific. Web.
Appendix A