Amino acids form proteins and are vital for the normal functioning of all human body systems. Lysine is an essential amino acid, meaning it is one of the building blocks of proteins and must be obtained from food sources. Lysine is critical in various bodily functions, such as growth, development, and energy production. This essay will discuss the chemical formula, the importance of lysine in the body, and its polarity.
The chemical formula of lysine is C6H14N2O2 (Figure 1), (PubChem Compound Summary, 2023). Lysine plays an essential role in the body, as it helps produce carnitine, which helps the body convert fatty acids into energy. It also helps produce collagen, a structural protein essential for healthy skin, hair, nails, and bones. Lysine also helps the body absorb calcium, essential for strong bones and teeth. This amino acid has a polar structure with both a positively and negatively charged end. The positively charged end is attracted to water molecules, while water molecules repel the negatively charged end. This polarity helps lysine attach to other molecules, such as proteins, essential for biological functions. Lysine also plays a vital role in the immune system, as it helps the body fight off viruses and bacteria. It is also essential for hormone production, as it helps the body produce hormones such as testosterone and estrogen.
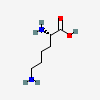
In conclusion, lysine is an essential amino acid that plays a critical role in the body. Its chemical formula is C6H14N2O2, and it is polar. Lysine helps the body produce energy, build proteins and fight off infections. It is also essential for hormone production and absorbing calcium. Since the human body cannot synthesize lysine, consuming products containing this amino acid is necessary.
Reference
National Center for Biotechnology Information (2023). PubChem Compound Summary for CID 5962, Lysine. Web.