Oxidation and reduction are typical reactions in several synthetic organic processes. Oxidation entails a rise in oxygen, a decline of hydrogen in the molecule, a rise of any atom electronegative more than carbon, or electrons loss in a reaction. Oxidation of aldehydes and alcohols may be carried out by a range of oxidizing agents comprising chromic acid, sodium dichromate, hydrogen peroxide, pyridinium chlorochromate, nitric acid, and sodium hypochlorite. Benzaldehyde may be oxidized efficiently to the benzoic acid by applying potassium permanganate (KMnO4) under basic conditions. This reaction entails benzaldehyde oxidation by the MNO4- anion that reduces to manganese dioxide. As potassium permanganate oxidizes the benzaldehyde, Mn7+ will be reduced to Mn4+ which precipitates the MnO2, a brown solid to be visible when the reaction progresses. Sodium hydroxide offers the alkaline conditions critical to complete the reaction.
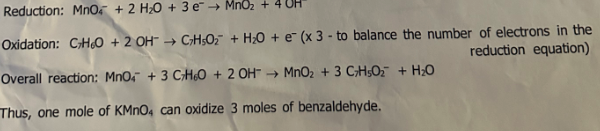
Benzaldehyde is oxidized to benzoate ion under basic conditions. Therefore, hydrochloric acid (HCl) is added to re-protonate the benzoate anion and form benzoic acid that precipitate out of the solution. The acid was recrystallized to remove insoluble impurities using water as the recrystallization solvent. The vacuum filtration is used to collect the acid and then wash the benzoic acid crystals using water to eliminate salts. The acid is then dried carefully in a desiccator up to the next lab time. Weighed the dry product and determined its percentage to be 79% yielding 0.93mg white crystalline solid and its melting point was 117oC after recrystallization. Treating benzoate ion with HCl to generate benzoic acid is not recrystallization but it is forming the insoluble form of benzoic acid.
Recrystallization involves taking a solid chemical without changing its a chemical and dissolves it into a solvent to supersaturate the solution and cools it to form crystals. The percentage of impurities in the benzoic acid after oxidation was generally not higher than 10 percent and was specifically 8%. Pure benzoic acid based on the IR file spectrum is 122.4°C, but the range varies from 114 to 122°C.