Table 1 contains the data of direct measurements and indirect calculations for p-nitroacetanilide as a product of the EAS reaction of acetanilide. The melting point and practical mass were obtained instrumentally, whereas the theoretical mass was obtained by indirect calculations from the reaction equation. Acetanilide undergoes nitration by the following mechanism:
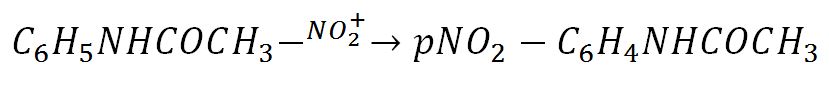
It is noteworthy that not only the para-substituted product, but also o- nitroacetanilide was isolated during this EAS reaction. However, unlike o-nitroacetanilide, p-nitroacetanilide appeared insoluble in alcohol, so it could be used for recrystallization and measurement of chemical purity. The mass of the acetanilide that entered the process was equal to 1.0060 g, from which the mole number could be determined by the following formula:
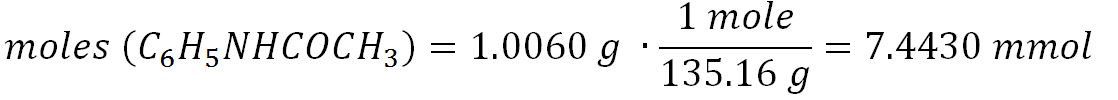
Proceeding from stoichiometric ratios, the amount of the resulting product is equal to half of the acetanilide entered, since a mixture of products is formed in the reaction. Then the theoretical mass of p-nitroacetanilide could be obtained by the following calculations:
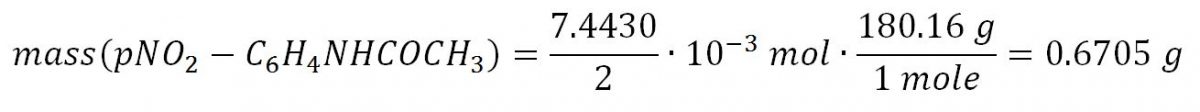
Then the percentage yield:
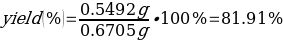
Table 1. Results. Instrumental measurements and indirect calculations for p-nitroacetanilide.
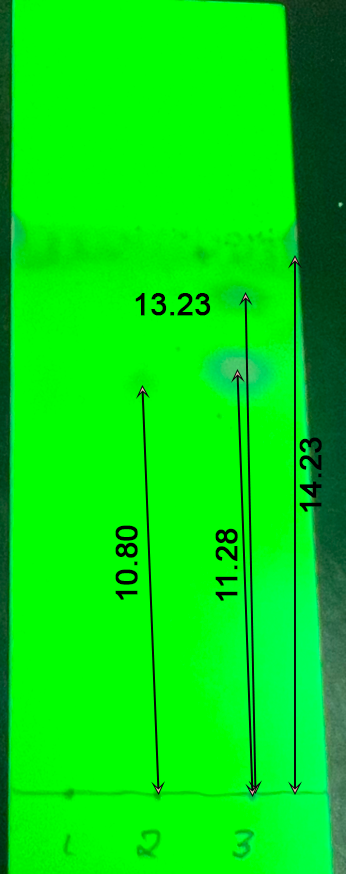
Three lines on the TLC plate were obtained for the product obtained, depending on the processing methods (Fig. 1). The first well loaded with crude p-nitroacetanilide showed no staining, and only for the second and third wells were the chromatographic results noticeable. In particular, two spots were characteristic of the third well, and only one little discernible spot for the second well. The calculated Rf coefficients are shown in Fig. 1.
Discussion
Nitration of aromatic compounds in the presence of sulfuric acid can proceed by electrophilic aromatic substitution mechanism, which results in the replacement of one or more hydrogen in the benzene ring. In the current experiment acetanilide was subjected to such nitration under the influence of a nitronium particle, which resulted in the formation of a mixture of two products, o-nitroacetanilide and p-nitroacetanilide. Substitution at the para-position is preferable for the electrophile because of the excess electron density centered on the -NHCOCH3 group. Recrystallization in ethanol was used to separate the mixture because o-nitroacetanilide is well soluble in alcohol.
As shown by the chromatography results, recrystallization can be considered a successful procedure for purification, since in this condition there was a spot for p-nitroacetanilide, but no spots for the product mixture, which is probably caused by the low content of p-nitroacetanilide in this mixture.
On the second and third wells, there were noticeable stains at similar distances, which may indicate the transfer of some of the product into the filtrate. The purity of p-nitroacetanilide can also be determined through the thermal characteristics of the product: the boiling point detected was not significantly different from the accepted values. Nevertheless, differences existed, which indicated the presence of impurities in the purified product.
It is more interesting to refer to the percentage yield as an indirect estimation of the success of the experiment. The calculations yielded a p-nitroacetanilide yield of 81.91%, which was justified by the presence of an impurity product as well as uncertainties in the measurements and experiment. Reference data show that in basic EAS acetanilide substitution reactions the yield of the main product can be as high as 90% versus 10% for o-nitroacetanilide. Such discrepancies can be classified as non-serious and justified by potential errors in the synthesis that do not affect the mechanism or by statistical error.
Other reasons could include competing reactions that were not taken into account, as well as minor sample loss during the laboratory procedures. In other words, the percentage yield is generally within the accepted values, which may also indicate that the experiment was successful.
References
- Zhao, H.; Bao, Y.; Farajtabar, A.; Jouyban, A.; Acree Jr, W. E. o-Nitroacetanilide Equilibrium Solubility in 15 Monosolvents: Experimental Determination, Mathematical Correlation, and Solvent Effect Examination. J. Chem. Eng. Data 2021, 66 (5), 2124–2133.
- 4′-Nitroacetanilide, 99%, Thermo Scientific™. Web.
- Sánchez-Viesca, F.; Gómez, R.; Berros, M. Theoretical Advances in Aromatic Nitration. Mod. Chem. 2015, 3 (1), 9-13.