“Organic compounds are a large class of chemical compounds whose molecule contain carbon.” (Organic Compound para 1). But the exception is required for compounds such as carbonates, oxides of carbon and cyanides, allotropes of carbon, etc.
Cyclohexene and cyclohexanol are organic compounds
Cyclohexene is a hydrocarbon with a formula C6H10. This cycloalkene (hydrocarbon contain a closed ring of carbon atoms) is a colorless liquid with a sharp smell. Cyclohexene acts as a precursor for adipic acid, maleic acid. dicyclohexyladipate, cyclohexaeneoxide, furthermore it is used as a solvent.
Cyclohexanol is an organic compound with formula (CH)25CHOH. This molecule is related to cyclohexane (cycloalkane with a molecular formula of C6H12) ring with a replacement of one hydrogen atom by a hydroxyl group.

Preparation
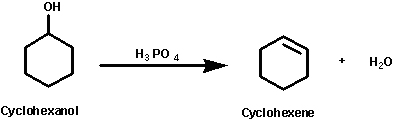
Functional group transformation is a major objective in modern organic chemistry this cyclohexene from cyclohexanol reaction belongs to a broad class of organic transformation called elimination reactions and with any strong acid can be used as a catalyst for example phosphoric acid sulfuric acid etc
Aim
This experiment demonstrates the synthesis of alkene from alcohol by acid-catalyzed dehydration.
Principle
It is a kind of elimination reaction where the hydroxyl group is removed from cyclohexanol by dehydration under the acidic condition to prepare cyclohexene, were phosphoric acid acts as a catalyst
Procedure
- Take a round-bottomed quick-fit flask.
- Measure 9 – 10ml of cyclohexanol. And Add to the round-bottomed Quik fit flask
- When we are processing the test the amount of alcohol used should be accurate
- Take a measuring cylinder
- Measure 3 ml of conc phosphoric acid
- Mix it properly by swirling it properly
- Add an anti-bumping granule to the flask and set it up in the electric heating mantle with a reflex condenser fixed vertically to the top
- Boil the mixture by heating
- We should adjust the heat control so that the mixture will boil for 10-15 minutes
- The next step is a distillation of the crude product
- Keep the whole apparatus for some time to come to a normal room temperature.
- Take the cooled mixture and rearrange the apparatus.
- The next step is a distillation of crude product for this purpose rearrange the instrument and connect it with a round-bottomed flask through the condenser
- Collect the distillate in the round-bottomed flask through the round-bottomed flask.
Separation
One has to ignite the heating device and boil the mixture gently for the entire cyclohexene vapor to condense in the condenser. Subsequently, he or she can collect the distillate which will be in the range of between 70 and 90°C. Once this is done, the heater can be switched off.
Purification
- Pour the distillate into a separating (tap) funnel and shake it with an equal volume of saturated sodium chloride solution (this has a higher density than ordinary water and allows the two layers to separate more easily).
- Once the two layers have separated, run off the lower aqueous layer and discard it. Pour the organic top layer (cyclohexene) into a small conical quick-fit flask and add a few granules of anhydrous calcium chloride (a drying agent).
- Stopper and swirl the flask gently until the liquid is clear. (The flask may be left overnight if desired).
- “Decant the cyclohexene from the conical flask into a clean round-bottomed flask and re-distill it, collecting the product in a previously weighed, clean, conical quick fit flask. Collect the liquid which comes over between 81 and 85°C (the boiling point of cyclohexene is 83°C).” (AS Experiment 12.3 (2): Preparing Cyclohexene from Cyclohexanol 1).
Works Cited
AS Experiment 12.3 (2): Preparing Cyclohexene from Cyclohexanol. 2009. Web.
Organic Compound: Overview. Absolute Astronomy. 2009. Web.