Introduction
When we compare the availability of plant nutrients in the soil, phosphorus turns out to be the least transportable and accessible to plants as compare other major plant nutrients in most soil states.
Much as phosphorus is plentiful both in its inorganic and organic state in the soil, it has pointed out that in most situations is major principal limiting factor that inhibits plant growth. According to Khan, Zaidi & Wani (2006)”the bioavailability of soil inorganic phosphorus in the rhizosphere varies considerably with plant species, nutritional status of soil and ambient soil conditions” (p.1).
To avoid situations where we have phosphorus scarcity, phosphate-solubilizing microorganisms or bacteria (PSM) are used to that they provide phosphate nutrients to vegetation in a more atmospherically-pleasant and sustainable way. Khan, Zaidi & Wani (2006) points out that:
The solubilization of phosphatic compounds by naturally abundant PSM is very common under in vitro conditions; the performance of PSM in situ has been contradictory. The variability in the performance has thus greatly hampered the large-scale application of PSM in sustainable agriculture.
Numerous reasons have been suggested for this, but none of them have been conclusively investigated. Despite the variations in their performance, PSM are widely applied in agronomic practices in order to increase the productivity of crops while maintaining the health of soils.
This review presents the results of studies on the utilization of PSM for direct application in agriculture under a wide range of agro-ecological conditions with a view to fostering sustainable agricultural intensification in developing countries of the tropics and subtropics. (p.2).
Generally, phosphorus is seen as a limiting nutrient that is responsible for causing lakes and reservoirs eutriphication. Due to the introduction of the controlling agencies, the sources that introduce phosphorus into the atmosphere (for instance we have the man induced and natural ones, the man induced comprising of fertilizers by farmers, wastes from industrial and domestic sewage and urban run off while the natural ones include the water fowl waste, atmospheric deposition, plant decomposition and weathering) have been reduced.
This has been done through the introduction of waste water treatment plant which has also contributed to the reduction of the total amount of phosphorus produced thus improving water quality.
In this case we it is worthy pointing out that Phosphorus is a very important nutrient which not only acts as a compound for the many plant structures but it also acts as a catalyst during the conversion of biochemical reactions in plants (Strickland & Parson, 1972). It can also be found as a free ion in different forms which includes water sources mostly found as salt in terrestrial environment; it is also found in detergents because it acts as water softeners and sometimes it too can be established in rock deposits.
Phosphorus nutrient plays a vital role of detaining and translating the sun’s energy to important plant compounds which acts as major growth limiting nutrients that promotes root development, enhances the formation of flower and seeds, it also plays an important role in the strengthening of the stems and stalks of a plants, facilitates crop maturity and production and finally it raises the resistance of plant diseases making them healthy.
The nutrient is an important component of DNA and RNA for all living things this is so because the structure grasping both the DNA and RNA are connected by phosphorus bond.
Its importance is seen in ATP where by it acts as the plants energy unit that forms during photosynthesis and this entails phosphorus in its entire structure. Therefore phosphorus is an important nutrient for the general vigor and health of all plants this is so because Phosphorus deficiency in plants can be identified by stunted plants during early growth or abnormal discoloration can be noticed (in plants, usually turns to dark bluish-green in color leaves and stems turning purplish).
Due to its high mobility level it turn out to be an advantage because when there is inefficiency of the phosphorus nutrient in a plant it can lead to translocation of the nutrient from an old plant tissue to younger growing areas.
Phosphorus nutrient content in most surface soils is very low compared to other nutrients like nitrogen and potassium and it also varies according to the variation in the soil types while phosphate which acts as limiting factor in the ecosystem on the plants occurrence has minimal effects on the plants (Tate, 1984).
Soil phosphorus is mainly affected by many factors which include; the parent material from which the soil is derived from, experienced climatic condition during soil formation, occurrence of soil erosion, phosphorus fertilizer application, and the degree of weathering.
Mainly, Soil phosphorus can be classified into two groups which includes; organic and inorganic phosphorus. Organic phosphorus is found in manures, microbial tissues and sometimes in the plant residues. Soils with low organic matter content contain 3% of organic phosphorus. But soils with high organic matter content contain 50% or more organic phosphorus. Inorganic soil phosphorus entails apetite, iron and aluminum phosphates and phosphorus on clay particles.
Soluble phosphorus in the soils reacts with soil compounds and then is converted to less available forms through phosphorus fixation. Due to these process phosphorus moves very little making it to stay closer to its place of origin as a result little phosphorus is lost through leaching. Phosphorus that is available in each soil varies as a result of the following factors:
Organic matter
Soils containing high volume of organic matter has considerable amount of organic phosphorus that are mineralized so that it can provide available plants with phosphorus. Presence of organic matter in the soils also acts as a chelating agent that combines with iron to prevent the formation of insoluble iron phosphates.
Application of heavy organic material for example plant residue, manure or green manure to soils with high pH value supplies makes the phosphorus and lead that are available in the soil to decompose which later provides acidic compounds that increases the availability of phosphorus in the soils (Tate, 1984).
Soil temperature
The rate at which plants absorb phosphorus is decreased by poor soil aeration and low soil temperature. Fertilizer which contains water soluble phosphorus is at a high level of increasing crop growth when the experienced weather is cool. A lot of soil moisture and compaction reduces the level of oxygen supply to the soils and this leads to decreased ability of plant roots in the absorption of soil phosphorus.
Compaction leads to reduction in the soil aeration and root zone pore spaces and this can later lead to the level of plant growth being reduced and at the same time the uptake of the phosphorus nutrients lowers. Finally compaction can decrease the total soil volume that plant roots penetrate through therefore affecting the total phosphorus that plants take in.
Type of clay
Type of the clay present in the soils tends to determine the rate at which phosphorus can be fixed in that soil. For example, clay particles retain or fix phosphorus in soils. However, Fine textured soils for instance clay loam soils tend to have greater fixing capacity compared to the one containing sandy coarse textured soils.
There is evidence that Soils that occurred during rainy season, or periods of high temperatures contains clays with large volumes of kaolinitic clays making them to have higher phosphorus fixing capacity compared to those that occurred during dry season and periods of low temperature which contains vermiculite making them to have lower fixing capacity (Tate, 1984). High temperature and rainfall also increases iron and aluminum oxides in soils that highly contributes to the added phosphorus in the soils.
Application timing
Soil phosphorus fixation increases proportionally with the time of contact between the soil particles and the soluble phosphorus. However the good utilization of this fertilizer phosphorus can be seen when the fertilizer is applied to the soils shortly before the plant is planted.
This practice can only be effective on those soils that have high phosphorus fixing capacities (Khan et al., 2007). In some areas for instance the coastal plains the fertilizers can be applied several months before the planting season and this will lead to little or no decrease in the amount of fertilizer phosphorus found in the soil.
Other nutrients
Other plant nutrients that are present in the given soil lead to the increase in the absorption level of phosphorus from soil. For example when ammonium fertilizer is applied at the same time with phosphorus to the soil, the rate at which the phosphorus uptake from the soil will be higher compared to applying phosphorus fertilizer alone.
Solubility of Phosphate in the Soil
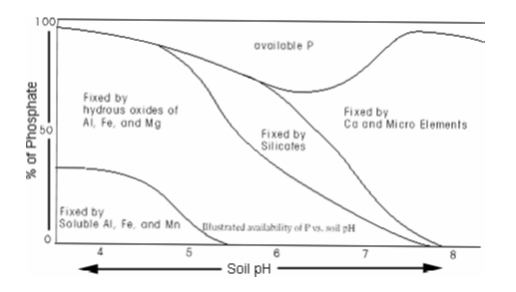
Phosphorus element is proved to stable or in other terms not easily soluble in most soils. This characteristic of phosphorus is attributed to the fact that the element is highly reactive and therefore reacts with other compounds readily available in the soil to very stable compounds further increasing the solubility of phosphate in the soil. Phosphate nutrients are absorbed by plants different chemical forms depending the acidity and basicity level (pH) of the soil and to a significant degree the organic materials.
From the diagram below, when the pH of the soil is between 4.0 and 5.0 phosphorus nutrients are taken in as phosphoric acid (H2PO4) and when the pH level in the soil is more than 5.0, phosphorus is taken in phosphite acid (HPO42-). On the other hand, when the pH level falls between 5.5 and 7.5, phosphorous absorbed as an amalgamation of the above two. The element is also sometime absorbed in the form of organic compound most of the time at the root interface.
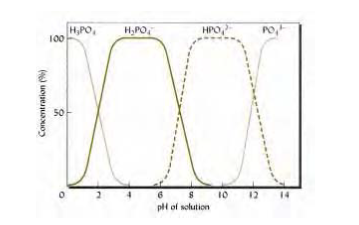
Research suggests that a lot of phosphate is readily accessible when the pH level is between 6.0 and 7.0. Therefore as the pH level of shifts from both end to neutral point, the calcium phosphate or ferrous-phosphate increases in solubility. In addition to this, the solubility of phosphate compounds will change with time.
Take for example when a fertilizer that contains phosphate as an active ingredient particularly between pH 5.5 and 7.5, the phosphate will begin to bond with elements such as iron and aluminum even though any phosphate molecules near plants will be absorbed immediately until the time
For example solubility of organic phosphorus that occurs in aquatic systems can be regulated by physic-chemical characteristic of water column. This can be witnessed through carrying out solubility experiments using different medium (Cardoso, 2002).
For instance when you want to carry out an experiment so that you can find out the effects of pH and Calcium on inorganic phosphorous, you will come to notice that this two elements affects the solubility of phosphorous differently. The results that you will come up with will show you that the solubility of phosphorous in water when the pH is above 8.5 will be high and when the calcium level is low at the PH of 9.0 it will not be affected. Phosphorus solubility decreases when calcium and PH level increases.
Mechanisms of Phosphate Solubilisation
In the mechanization of phosphorus solubility, Bacterial species which contains mineralization and solubilization potential for both organic and inorganic phosphorus respectively (Hilda & Fraga, 2000; Khiari & Parent, 2005) is usually used.
The rate at which the solubilizing activities occurs is determined by observing the rate at which microbes release metabolites like organic acids , through this hydroxyl and carboxyl forms a chalate group that forms a cation bond to phosphate that is later converted to soluble forms. Phosphate solubilization occurs as a result of different microbial processes/mechanism which entails organic proton extrusion and organic acid production. (Surange, 1995; Dutton& Evans 1996; Nahas, 1996).
A large number of saprophytic bacteria and fungi that acts on soluble soil phosphates can be used to carry out phosphorus solubilization test mainly through chelation-mediated mechanism (Whitelaw,2000). Solubilization of inorganic phosphorus occurs when the actions of organic and inorganic acids that is secreted by phosphorus solubilizing bacteria decreases the soils basic pH (Kpomblekou &Tabatabai 1994; Stevenson, 2005).
Soil phosphorus can be dissolved by the phosphorus solubilizing bacteria by means of the production of low molecular weight compounds of organic acids mainly keto gluconin acids and gluconic acids in addition to lower rhizosphere’s PH the biotical production of proton is used.
The solubilization ability of phosphorus solubilization bacteria correlates with the PH of the medium. Potassium concentration in the soil solution is altered by the release of the root exudates like the organic ligands. Phosphorus solubilizing bacteria produces organic acids which lowers the phosphates PH through solubilization (Strickland & Parson, 1972).
Phosphate Solubilising Bacteria
The occurrence of Phosphorus solubilizing bacteria that is naturally evidenced by rhizospheric phosphorus solubilizing microorganism has been there since 1903 (Khan et al., 2007). In phosphorus solubilization, bacteria are more effective compared to fungi. In soil population, the phosphorus solubilizing bacteria consists 1 to 50% compared to phosphorus solubilizing fungi which consists of 0.1 to 0.5 %( Chen et al., 2006).
In the soil bacterial community, ectorhizospheric strains from Pseudomonas, endosymbiotic rhizobia and Bacilli are among the most effective phosphate solubilizers. The most powerful phosphorus solubilizers include pseudomonas, Bacillus, Rhizobium and enterobacter (Whitelaw, 2000) and the most important strain includes Bacillus, megaterium, B.circulans, B.Polymyxa, pseudomonas striata among others.
Phosphate rock has minerals which most of the time are detected to be insoluble and this makes them to lack the ability of supplying crops with enough phosphorus for it to use and as a result of this, there is a phosphorus solubilizing bacteria which is mostly used to increases the crop yield up to 70% (Verma, 1993) combination of inoculation and arbuscular mycorrhizia together with phosphorus solubilizing bacteria which promotes the uptake of native phosphorus from soil together with those that comes from phosphatic rock (Goenadi et al., 2000; Cabello et al., 2005).in addition, it should be noted that high crop production mostly comes as a result of phosphorus which is fixed in the soil during solubilization and those phosphate that are applied by the phosphorus solubilizing bacteria.
Those microorganisms that have phosphate solubilizing potential usually increase the presence of soluble phosphates and this promote the rate at which plant grows through the improvement of the biological nitrogen fixation. Solubilizing bacteria also enhances total number of nodules, increases the dry weight of the nodules, increases the component yields and grain yields and also the level of nutrient accessibility and uptake is improved in some crops for example, Soybean ( Son et al., 2006).
In some crops the bacteria enhances the seedling length, increases sugarcane production. Bacteria application increases biological yields while when the same bacteria are applied with mycorrhizae the maximum grain weight is achieved. When single or dual bacteria are inoculated with phosphorus fertilizer the grain yield of wheat is improved.
Mycorrhiza and Pseudomonas Putida increases the leaf chlorophyll level in the barley crops. Rhizospheric microorganisms positively interact in the promotion of plant growth and it also enhances the uptake of nitrogen and potassium. It also increases the yield of green grams. From this it can be noted that integration of phosphorus with biofertilizer provides the crop yield with full fertilizer rate and reduced use of fertilizer minimizes cost of production and maximizes net return (Jilani et al., 2007).
Phosphorus compounds which is known as ortho-phosphate is a limiting nutrient in most flowing and stagnant waters(Correll, 1998) Increased concentration of phosphorus can be caused by higher input of waste water, avulsion and others which can result to eutrophication of water and this can lead to oxygen depletion, increased algae growth et cetera.
Phosphates seep into the water systems from fertilizers, rocks and soils. Phosphate salts are also introduced into rivers and lakes when there is a natural event for example; heavy rainfall that wash off topsoil from agricultural fields (Sissingh, 1983).
This activities results to increased phosphate concentration that cause algal bloom and this ends up affecting the organisms that are found in rivers and fish kills. When untreated waste water is released into the environment due to its content of phosphates from detergents can also cause same impacts. Measures can then be taken to lessen the inputs and prevent destructive impacts that can take place as a result of eutriphication.
Phosphorus can be eliminated in wastewater so that it can meet the needed limits of phosphorus concentration in the effluent, this can be done using the following methods; Biological elimination of phosphates which entails the integration of phosphate in microbial biomass and the second method is chemical-physical elimination of phosphates which entails the chemical precipitation of ortho-phosphates using metallic salts
Methods for Determining Phosphate
Phosphate or phosphorus concentration can be determined by using different methods, this includes Molybdenum blue method and Vanadate/ Molybdate method also known as yellow method (Robinson & Sharpley, 1994). In molybdenum blue method an acidic medium, ortho-phosphates bond with ammonium molybdate are used to form molybdenum phosphoric acid.
Then there is molybdate method which occurs by acids reacting with ammonium vanadate to form ammonium phosphoric. The two above mentioned methods are all about measurement of orthophosphate. in addition a sample must be attained and it should be unfiltered.
The most commonly used method is t what the one that is commonly referred to as the Johnson and Ulrich’s procedure whereby selenium chloride(SnCl2) and ascorbic acid s employed as a reducing agent in the process. In this process, once the sample is extracted, 5ml of the aliquot of the resulting extract is then diluted with 15ml of distilled water so that the final solution is 20ml. after this, 2ml of the result solution after dilution is then put in a test tube followed by adding of ascorbic acid.
The resulting test sample is then given approximately 10 minutes to rest until there is a visible color before the absorbance is determined at 880nm wavelength using Beckman spectronic 20. The obtained curve is then compared with the standard phosphate curve whose phosphate concentration is between 0.5 and 5ppm.
Laboratory Experiment: Quantitative Analysis of the Phosphate Solublising Ability of Endophytic Bacteria
This set of laboratory experiments was aimed at determining how different medium affects bacteria growth. Furthermore, the experiment was purpose at finding out whether endophytic bacteria can liquefy phosphate from an insoluble source and then quantifying the amount of phosphate discharged into growth medium by the endophytes.
Finally, from the collected information in the first three objectives, the experiment also sought to optimize the technique employed in quantifying the amount of phosphate discharged. This experiment measures how different set of medium (environmental factors such as endophytes) affect bacteria growth. This was achieved by manipulative the conditions of the environment in which bacteria strains were put and the observed to see their growth. Different parameters such as pH were also determined.
Three different types of mediums were prepared followed by culturing each of the four bacterial on the mediums and then the degree of growth of bacteria strains in different mediums tabulated. By knowing which medium favors optimum growth, we are able to apply it in our daily life applications particularly in managing fungal and bacterial growth. Instead of using chemicals to control bacteria, we can apply unfavorable medium that will kill these bacteria.
According to Schulz & Boyle (2006) “endophytes are self-perpetuating naturally occurring fungi living symbiotically in certain species of grass and they produce certain natural byproducts that are of significant benefit to their host plant” (p.2). These bacteria’s help plants in surviving. It has being observed in general that endophytes improved turf grass particularly were research was being carried out and increased the quality of plant with time.
Autoclaving Machine
This machine was use to sterilize the apparatus that were being used. Most of biological and chemical solutions used in the laboratory are sanitized to eliminate any possibility of fungal and microorganism development when these solutions are stored.
Autoclaving technique is a preferred method when of sterilizing buffer solutions because it is less time- consuming and slightly affordable. Before any solution is put into the autoclave machine, care is normally taken not to insert those solutions that contain heat sensitive nutrients. Another care that must be taken is the material used; these include all materials made from borosilicate glass or any material classified as autoclavable plastics.
Typical, the machine heats solution contents to approximately 1210C which in principle in relation to water, it is 21oC above. To stop the sample solution from boiling over/vaporizarion, Judelson (2004) points out that the machine chamber must be pressurized when carrying out the procedure. Once the sample is loaded and the autoclaving machine started, the time taken for sterilizing the solution is taken after the machine attains it optimal operating conditions which is 121o C/ 15 p.s.i and not from the time the start button was pushed.
Ion chromatograph
According to Scott (2011), “ion chromatography is a form of liquid chromatography where retention is predominantly controlled by ionic interactions between the ions of the solute and counter ions that are situated in, or on, the stationary phase” (p.1).The technique of ion chromatography is commonly employed in analyzing water by scientists.
By applying the technology of ion chromatograph, we are in a position to determine concentrations or quantity of key anions, like nitrite, fluoride, nitrate and chloride anions. In addition to anions, the technique is also widely used in determining the concentration of cations for instance the concentration of lithium, magnesium, ammonium and sodium in up to micro-units such as parts-per-billion (ppb) (Bruckner, 2011).
In principle this type of liquid chromatography assesses the quantity of ionic species in a given solution depending on the way ions in the solution interact with the resin. Separation of these ions is solely determined by size of the ions and type of species. Solution is injected into a chromatograph column that is under intense pressure where ions in this solution depending on their nature and size are absorbed on the column lining.
As a result of these ions being attached to the lining couple with passing the eluent liquid, ions that are absorbed to the lining begin to separate into distinct groups depending on their properties. The amount of time that any species is retained i.e. retention time is dependent on the amount of ions in sample under consideration (Bruckner, 2011).
Sample Preparation and Collection
Before injecting any liquid into the column, it must be filtered to eliminate any particles and other contaminates that may lead to microbial modification. All collections are done using a sterilized syringe. The collected sample is normal kept at low temperatures for further analysis.
On the other when analyzing solid samples, normally water or acidic solution is used. According to Bruckner (2011) “the elution time, or time it takes for the ion to move through the column, varies for each ion species as they elute from the column separately as the pH and/or ionic strength of the eluent is increased”(p.3). Once the analysis is done and the graphs obtained, the areas under the peak represents the concentrations of those ions. Smaller peaks represent minimal concentration while bigger peaks represent higher concentration.
Objectives
- This set of experiment was aimed at finding out how different medium affect bacteria strains growth.
- To find out whether endophytic bacteria can dissolve phosphate from an insoluble source.
- To find out the quantity of phosphate discharged into growth medium by the endophytes.
- To optimize the technique employed in quantifying the amount of phosphate discharged.
Materials/Apparatus and Experimental Procedure
List of Compounds
- 2-sucrose asparagines agar solid medium (SA).
- Acinetobaler SP (R213).
- Agar 1.
- Ammonium sulphate (NH4)2SO4.
- Glucose.
- Magnesium chloride (MgCl2.6H2O).
- Magnesium sulphate (MgSO4.7H2O).
- National botanical research institutes phosphate growth (NBIRP).
- Pantoea agglomerans (S222),
- Potassium chloride (KCl).
- Pseudomonas fluorescens (L228).
- Pseudomonasveronii (L23).
- Solid medium.
- Sucrose and 3-nutrient agar solid medium (NA).
- Tri Calcium Di-Phosphate (TCP).
List of Apparatus
- Autoclaved blue cap bottles 500ml
- Autoclaving machine
- Ion chromatograph
- Oven
- Pension burner
- pH meter
- Phosphate detectors
- Spatula
Experimental Procedure
Biological Methods
Bacteria strain culturing: First, three different kinds of mediums were prepared followed by culturing each of the four bacteria strains in the prepared medium. The cultured bacteria strains were kept warm at 30ºC. As a precautionary measure, the plates were wrapped in a laboratory film (para film) which prevented the medium from drying as a result of heat. The degree of the bacteria growth was observed and this level tabulated.
Solid Medium Preparation: Agar 1 medium was cooled and then poured into agar plats. This was then followed by weighing 2.5g of Tri Calcium Di-Phosphate (TCP),7.5 g of agar 1, 0.1 gram of potassium chloride(KCl), 5 g of glucose and 0.06 gram of ammonium sulphate (NH4)2SO4. The weighed reagents were then poured in a 500ml blue cap bottle which contained 50 ml of deionized water. This solution was diluted to 400 ml. the resulting medium solution was then autoclaved for 20 minutes at 121ºC then given adequate time to cool to facilitate shift operation.
In a separate 100 ml blue cap bottle containing 20 ml of deionized water, 5 grams of magnesium chloride (MgCl2.6H2O) was dissolved in it and then the solution diluted to 100ml. the resulting solution was then autoclaved for 20 minutes at 1200C. 0.25 gram of magnesium sulphate (MgSO4.7H2O) was treated the same way as magnesium chloride. 50 ml of each of the above two autoclaved solutions was added to the earlier prepared 400 ml solution making the total volume of the medium to be 500 ml. With a pension burner on to prevent contamination, this solution was poured into agar plats. These plats were labeled and the date written on them before being kept in the fridge.
2- Sucrose Asparagines Agar Solid Medium (SA) Preparation: 7.5 gram of agar 1 and 10 grams of sucrose was weighed and then dissolved into deionized water into contained in 500ml sterilized blue cap and then the solution diluted to 500 ml. the resulting solution was then autoclaved at 121 ºC for 20 minutes. After autoclaving, the medium was cooled and then poured onto the agar plats under the watch of Pension burner was used to prevent any contamination may occur.
3-Nutrient Agar Solid Medium (NA) Preparation: 14 grams of nutrient agar was weighed and then dissolved in 100 ml deionized water contained in 500 ml sterilized blue cap bottle and then the solution diluted to 500 ml. this was followed by autoclaving the resulting in solution medium for 20 minutes at 121ºC and final poured onto agar plates after cooling.
Autoclaving Process
Once the sample is loaded and the autoclaving machine started, the time taken for sterilizing the solution is taken after the machine attains it optimal operating conditions which is 121o C/ 15 p.s.i and not from the time the start button was pushed. The number of autoclaving was set to 6. Each specimen was put and waited for 20 minutes. It was turned off, given time to cool and then removed for the next step
Method 2: Broth Medium Preparation
National botanical research institutes phosphate growth and broth medium (NBIRP) were prepared as follows: 5 grams of glucose, 2.5 grams of Tri Calcium Di-Phosphate (TCP), 0.1 grams of potassium chloride (KCl), and 0.06 grams ammonium sulphate, (NH4)2SO4 were weighed and then dissolved in 50 ml deionized water contained in a 500ml blue cap bottles and then the solution diluted to 400ml (in this medium no agar was added).
The resulting solution was then subdivided into five 80ml and put in a sterilized flask. The result five medium (broth) was then autoclaved for 20 minutes at 121ºC. After 21 minutes, it was left to cool and then transferred for the next test.
5 grams of magnesium chloride (MgCl2.6H2O) were dissolved in 20ml of deionized water contained in 100ml sterilized blue cap bottle and then the solution diluted to 100ml. this was then autoclaved for 20 minutes at 121ºC. The same preparation procedure was repeated for 0.25 grams of magnesium sulphate (MgSO4.7H2O).
Finally 10ml of each of the two that is magnesium sulphate and magnesium chloride were then added to each of the five flasks that contained 80 ml broth medium making the final volume of the new broth to be 100ml.
Chemical Procedures
Method 3: Ions Chromatograph
Three samples were made up to 500 ml of double deionized water, then these samples were run in ion chromatography the reading before the samples were autoclaved and also after three different times after autoclaving. The pH of the solution was also taken and recorded.
- TCP 2.5 g of TCP was weighed out dissolved in about 50 ml double deionized water and made up to 500 ml.
- (NBIRP) no TCP or agar was added
- Double deionized water
Method 4
0.5 grams of TCP was weighed and then dissolved in 100ml double deionized water contained in the volumetric flask.
pH Calibration
The five specimen broth mediums were run through ion chromatograph and results tabulated. The pH of the solution was also taken and recorded. This was followed by culturing the first four broth medium with 80ml of broth medium as shown below:
Table 1: culturing of medium.
Results
The table below gives symbols that are used.
Determination of phosphate solubilisation on solid medium over time.
Table 2: Table showing the solubilisation of phosphate solid medium over time.
Table 3: Table showing the growth of the bacteria strains after four days incubation at 30°C.
In this table 3 the more the + symbol the more the growth over that given medium. In addition, the symbols – stand for no growth. We see that within four days Pseudomonas fluorescens do not grow in all three medium within four days while none of the bacterial strains showed any growth in Nutrient Agar solid medium (NA). These microorganisms that grow in Tri Calcium Di-Phosphate (TCP) will be able to use phosphate nutrients that solubilises after three days when incubated at 30oC over solid medium.
Table 4: Table show the growth level of the bacteria strains after six days incubation at 30°C.
From table 4 we see that all the bacteria strains namely; pantoea agglomerans, pseudomonasveroni, acinetobaler SP and pseudomonas fluorescens grew in Tri Calcium Di-Phosphate after six days with yellow fluorescence being observed in pseudomonasveroni and pseudomonas fluorescens. This is attributed to high amount of phosphate that was discharged in the solid medium.
Unlike from table 3 where there was no growth of pseudomonas fluorescens bacterial strains in Tri Calcium Di-Phosphate (TCP) medium, we see that after six days, there was good growth of pseudomonas fluorescens in Tri Calcium Di-Phosphate (TCP) medium with yellow fluorescence.
Still from table 4 after incubating at 30°C for six days, Pantoea agglomerans, pseudomonasveroni and acinetobaler SP bacterial strains showed some good growth in solid agar (SA) except for pseudomonas fluorescens which showed no growth at all. Finally still from table 4 we see that even after six days of incubation at 30°C, none of the of the bacteria strains microorganism grew in nutrient agar solid medium (NA).
This can be attributed to the factor that there was no solubilisation of phosphate ions in agar solid medium (NA), a crucial nutrients for growth. From these tables we can conclude bacterial strains growth can be optimized when we incubate these microorganisms for six days at 30°C in a given medium.
In particular, good results are achieved when we incubate these bacteria strains microorganisms in Tri Calcium Di-Phosphate (TCP) medium for 6 days at 30°C. On the other hand, there is no growth for all bacterial strains microorganism in Nutrient agar solid medium (NA) and indication of no solubilisation of phosphate nutrients.
Method 3 results
Table 5: showing the amount of nutrients determined in the sample using Ion chromatograph 21/2/11 after 14 days.
Results Analysis
From table 2 we see thatmicro-organisms pantoea agglomerans (S222), pseudomonasveronii (L23), and pseudomonas fluorescens (L228) all showed good growth with 3 days when incubated at 30oC on 3-nutrient agar solid medium (NA). However, even though acinetobaler SP (R213) microorganism showed some growth on nutrient agar solid medium (NA) medium, the growth was poor.
This poor growth can be attributed poor solubilization of phosphate in the solid medium. When incurbated in 2-sucrose asparagines agar solid medium (SA) , all the three microorganism showed good growth. Pseudomonas veronii (L23) and pseudomonas fluorescens (L228) gave a fluorescent yellow pigment with 3 days of incubation on Tri Calcium Di-Phosphate (TCP) medium as Pantoea Agglomerans (S222) gave yellow pigment.
We see that within four days Pseudomonas fluorescens do not grow in all three medium within four days while none of the bacterial strains showed any growth in Nutrient Agar solid medium (NA). These microorganisms that grow in Tri Calcium Di-Phosphate (TCP) used use phosphate nutrients that solubilises after three days when incubated at 30oC over solid medium.
Still from table 3, we see some growth of bacteria strains of pantoea agglomerans, pseudomonasveroni and acinetobaler SP as represented by symbol + in the medium Tri Calcium Di-Phosphate (TCP) and solid agar. Again this growth is attributed to availability of phosphate nutrients that liquefied as within 3 days after incubation at 30oC from table 2.
Table 6: Table shows the IC and pH meter readings of NBIRP samples before autoclave 14/2/11 after 7 days.
Table 7: Table shows the IC and pH meter readings of NBIRP samples after autoclave.
To calculate the quantity of PO4 in fourth media we do as follows:
Actual amount of PO4
Molecular weight of TCP =309.8mole/gram
Molecular weight of PO4 =189.8
Weight of PO4 in TCP =Mw PO4/Mw TCP =189.8/309.8 = 0.612 mol/g
PO4 g in 500 ml = 0.612g *2.5g = 1.53g/500ml
PO4 g in 1 ml = 1.53g/500ml = 0.00306 g/ 1 ml
PO4 mg in 1ml = (3.06 mg /1 ml) * 1000
PO4 mg in Littre =3060 mg / Littre
PO4 ppm = 3060 ppm
2.5g of tricalicium dissolved in PO4/500ml. after running ion chromatograph phosphate ions and sulphate nutrients were detected in the solution all samples except in sample three. On contrary, chlorine ions were reported to in insignificant meaning they were adsorbed on the linings of ion chromatograph.
From tables 6 and 7, we see that autoclaving had no effect on phosphate (PO4) and sulphate (SO4). This is because the amount of phosphate and sulphate obtained before and after autoclaving remained the same with small deviation as a result of experiment errors in measuring even after 7 days of incubation.
When the bacterium was added the following results were obtained:
Table 8: Showing results after the bacteria was added.
From this table we see that when bacteria strains were added and incubated for seven days, the level of sulphate and phosphate went down. For chlorine ions, there was no significant change. We see that with time, the pH went down meaning the medium became more acidic as the amount of phosphate went up. Meaning the growth of bacteria strains also increased.
In the cause of carrying out this experiment we faced challenges such as some specimen not dissolving in the medium. In addition, the solution becomes more acidic with time which could have affected the medium. The amount of phosphate nutrients lost as a result of acidification can be represented by the following equation:
Ca PO4 + 2HCl CaCl2+ H2PO4
Conclusion
From these tables we can conclude bacterial strains growth can be optimized when we incubate these microorganisms for six days at 30°C in a given medium. In particular, good results are achieved when we incubate these bacteria strains microorganisms in Tri Calcium Di-Phosphate (TCP) medium for 6 days at 30°C. On the other hand, there is no growth for all bacterial strains microorganism in Nutrient agar solid medium (NA) and indication of no solubilisation of phosphate nutrients. The objectives of the experiment were met.
According to this research paper, we can conclude that total amount of phosphorus fertilizer added to the soils most of the time is affected three processes which includes the; chemical, biological and physical processes (Robinson & Sharpley, 1994). This process is applied during the application of these fertilizers to the soils.
Therefore it is very important that each and every person understands this processes in details so that he or she can have full information on how phosphate gets into the soils, their availability in soils, their solubility level, methods of determining the available phosphorus in soils and many others. By doing so and understanding them the farmers will make good use of this phosphorus fertilizer and this will improve their production.
It should also be noted that due to phosphorus being an important nutrient that is needed in sufficient level by the plants and all crop productions greatly depends on them. These can be evidenced where by when there is no intake of the phosphorus fertilizer by plants the total output will be lower compared to a case where there is sufficient intake and this should be enhanced to maintain the total soil phosphorus because not all the farmers can afford to buy this fertilizers.
As compared to the ever rising population we all need the presence of phosphorus fertilizer in the soils so that it can raise the level of productions to meet the needs of this population.
Phosphorus is a major macro-nutrient without which plants’ growth might be compromised. Therefore I would like to urge everyone to avoid those activities that release or induce a lot of phosphorus into the soils because we know that they lower the productions too. By doing so the soils will have enough phosphorus nutrients that are required by the plants which will later enhance production.
References
Biogro. (n.d). Phosphorus in soils. Web.
Bruckner, M. Z. (2011). Ion chromatography. Web.
Cardoso, I.M. (2002). Analysis of phosphorus by (PNMR)-P-31 in ox sols under agro-forestry and conventional coffee system in Brazil. Geoderma Journal, 112, 51-70.
Correll, D.L. (1998). The role of phosphorus in the eutrophication of receiving waters. A Review Journal Environ. Qual., 27, 261-266.
Judelson, H. (2004). Operation of the autoclaves. Web.
Khan, M., Zaidi, A. & and Wani, P. (2006). Role of phosphate-solubilizing microorganisms in sustainable agriculture -A review. Agronomy for Sustainable Development Journal, 27, 29-43.
Robinson, J.S., & Sharpley, A.N (1994). Organic phosphorus effects on sink characteristics of iron-oxide-impregnated filter paper. American Journal of Soil Science, 58, 58-761.
Scott, R.P. (2011). Ion chromatography. Web.
Sissingh, H.A. (1983). Estimation of plant-available phosphates in tropical soils. A new analytical technique. New York, NY: Prentice Hall.
Strickland, J.D.H., & Parsons, T.R. (1972). A practical handbook of the sea water analysis. Ottawa, OT: Prentice Hall.
Tate, K.R. (1984). The biological transformation of phosphorus in soil. Journal of Plant Soil, 76, 245-256.