Introduction
The half-life period is part of the process occurring with radioactive elements. The element decays into other elements during this period while emitting radiation, as shown in Figure 1 below. The half-life process is slow and can last up to thousands of years.
Radioactive carbon (C) is converted into various isotopes of nitrogen (N), emitting beta and alpha particles (Flowers 1032). The radiation emitted during the half-life can be used for the treatment of various diseases and other applied purposes. One of the most exciting examples of such a half-life is the relationship between radioactive carbon and nitrogen.
The Role of the Half-Life Period
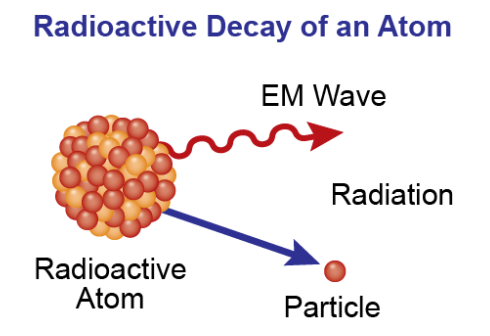
Radioactive carbon has several distinct properties, including its ability to decay and emit radiation, known as its half-life. Radioactive carbon turns into nitrogen, and the radiation emitted helps to control oxygen levels in the atmosphere. Radioactive carbon has a radioactive half-life of about 5.7 million years (Flowers 1034). This means that half of the initial dose of radioactive carbon will decay into nitrogen over this period.
The radiation emitted during the decay contributes to regulating the levels of oxygen and nitrogen in the atmosphere. Oxygen and nitrogen have different chemical properties, so their content levels in the environment must be balanced in a certain way. Radioactive carbon and its half-life help to establish and maintain this balance easily. As a result, the oxygen level in the atmosphere remains the same, which helps avoid negative consequences for animals, plants, and our environment.
Conclusion
In conclusion, the relationship between radioactive C and N is one of the most critical processes in nature. The half-life of radioactive carbon contributes to preserving the balance of oxygen and nitrogen in the atmosphere, which is necessary for maintaining life on Earth. Thus, radioactive carbon is a critical element in regulating atmospheric pressure and temperature, as well as the processes that support biodiversity on the planet.
Works Cited
Flowers, Paul. Chemistry 2e. OpenStax, 2019. Web.
“Medical Countermeasures for Radiation Exposure and Contamination” ORAU, n.d. Web.