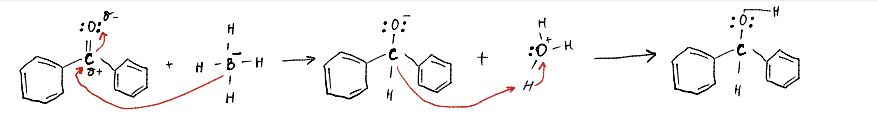
Description of the Mechanism
The reduction of Benzophenone involves the addition of a proton to the carbonyl group of the parent compound, which converts the ketone group of Benzophenone into a secondary alcohol (Benzhydrol). The reduction mechanism is realized by nucleophilic attack of NaBH4 (BH4- anion) in the presence of alcohol or water. The BH4- anion acting as a nucleophile attacks the carbonyl carbon of Benzophenone due to the presence of a partially positive charge zone, which leads to the breaking of the double bond, electron density shift to oxygen, and the formation of a negative charge, and the attachment of hydrogen to the carbonyl carbon. Such oxygen, acting as a nucleophile, then attacks the oxygen atom within the water or alcohol and strips off its hydrogen, which balances the higher electron density zone and leads to the formation of a secondary alcohol.
Discussion
The Yield Before and After Recrystallization
During the experiment, 202 mg (182.22 g.mol-1, 1.109 mmol) of Benzophenone was converted to 145 mg (184.23 g.mol-1, 0.787 mmol) of Benzhydrol before recrystallization and 128 mg (184.23 g.mol-1, 0.695 mmol) of Benzhydrol. Stoichiometrically, the two organic substances relate to each other as 1 to 1, so the expected molar yield of Benzhydrol was 1.109 mmol (204 mg), but the actual yield was 1.5-2 lower. Thus, the percentage yield of Benzhydrol before and after recrystallization was 72% and 63%, respectively. The loss of substance at both stages of extraction could be related to undesirable processes during synthesis, the use of non-sterile laboratory utensils, or experimental errors associated with inaccurate weighing.
Melting Point
The purity of the resulting product can be verified in two ways: by melting point examination and by spectroscopic instrumental analysis. On the one hand, the melting point of Benzhydrol before recrystallization was 58-60 °C, whereas, after purification, the melting point reached 62 °C. The increase in the melting point after recrystallization seems to be an expected result since the substance was purified of impurities. At the same time, the reference melting point for Benzhydrol is between 64 and 68 °C, indicating a lack of product purity and potential traces of impurities (Fisher Scientific, n.d.). The primary sources of contamination could be unwashed solvents or traces of unreacted Benzophenone.
Infrared and Nuclear Magnetic Resonance Spectrometry
On the other hand, an infrared spectroscopic examination may allow the identification of potential problems in product purity. Table 1 shows the characteristic bands for Benzhydrol obtained in the synthesis and the reference. The appearance of the band in the region of 1639 cm-1 may indicate the presence of Benzophenone residues in the substance since this peak corresponds to the extension of the carbonyl C=O group.
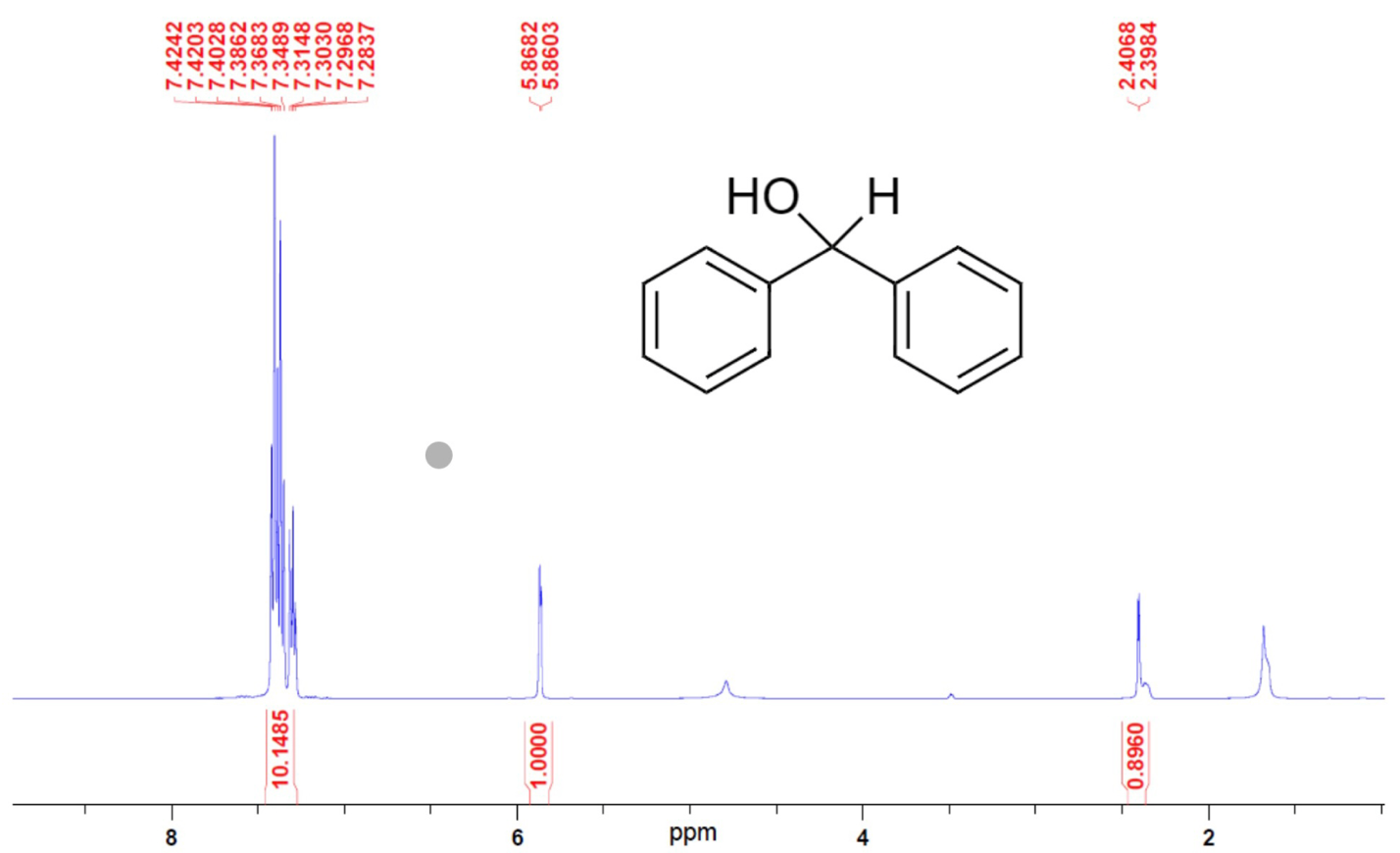
Figure 2 shows the 1H NMR spectrum for Benzhydrol; several distinct peaks are visible in this spectrum. The peaks in the region of 7.3 ppm correspond to ortho-protons of the benzene ring, and the peaks near 7.0 correspond to meta-protons. The signal in the region of 5.8 ppm corresponds to a hydrogen of the hydroxyl group unshielded due to the presence of neighboring oxygen, and the peak in the region of 2.4 ppm corresponds to an isolated proton on the hydroxyl carbon.
Table 1. Comparison of IR peaks for reference and experimental Benzhydrol
Reference
Fisher Scientific. (n.d.). Benzhydrol, 99%, thermo scientific chemicals. Fisher Scientific. Web.