Introduction
Water Holding Capacity (WHC) is an essential technological production parameter that determines the ability of chicken to absorb and contain moisture. In terms of consumer appeal, low WHC values give the chicken a visually deficient appearance, expressed as excessive pallor, inappropriate texture, and dry meat. For this reason, the study of patterns related to the moisture content in chicken is one of the critical objectives of production technology; one such predictor is the medium acidity index. In the present laboratory work, the main issue is to investigate the potential relationship between WHC as a measure of moisture content and chicken pH; specifically, the question is to identify the effect of meat pH on WHC. Determining the relationship between these two variables allows for a deeper understanding of processes to optimize production steps.
Results
Direct measurements for fifteen chicken meat samples yielded mass values (in grams) for both raw but acid-treated and cooked meat. Table 1 below shows the results of the direct measurements. For each sample (3 cm thickness), the weight is given both after treatment with acetic acid (column 4) and after cooking (column 5), as well as the original weight corresponding to the raw chicken disc before any interventions (column 3). In addition, the table contains information on the pH measurement for each of the chicken meat treatments, whether acidification or cooking in a water bath at 80°C. Notably, after treatment of the chicken meat samples with acetic acid, the mass of each disk increased by an average of 3.026 g, which may indicate that the samples absorbed liquid over time, with the maximum increase in mass being true for the maximum acid concentration.
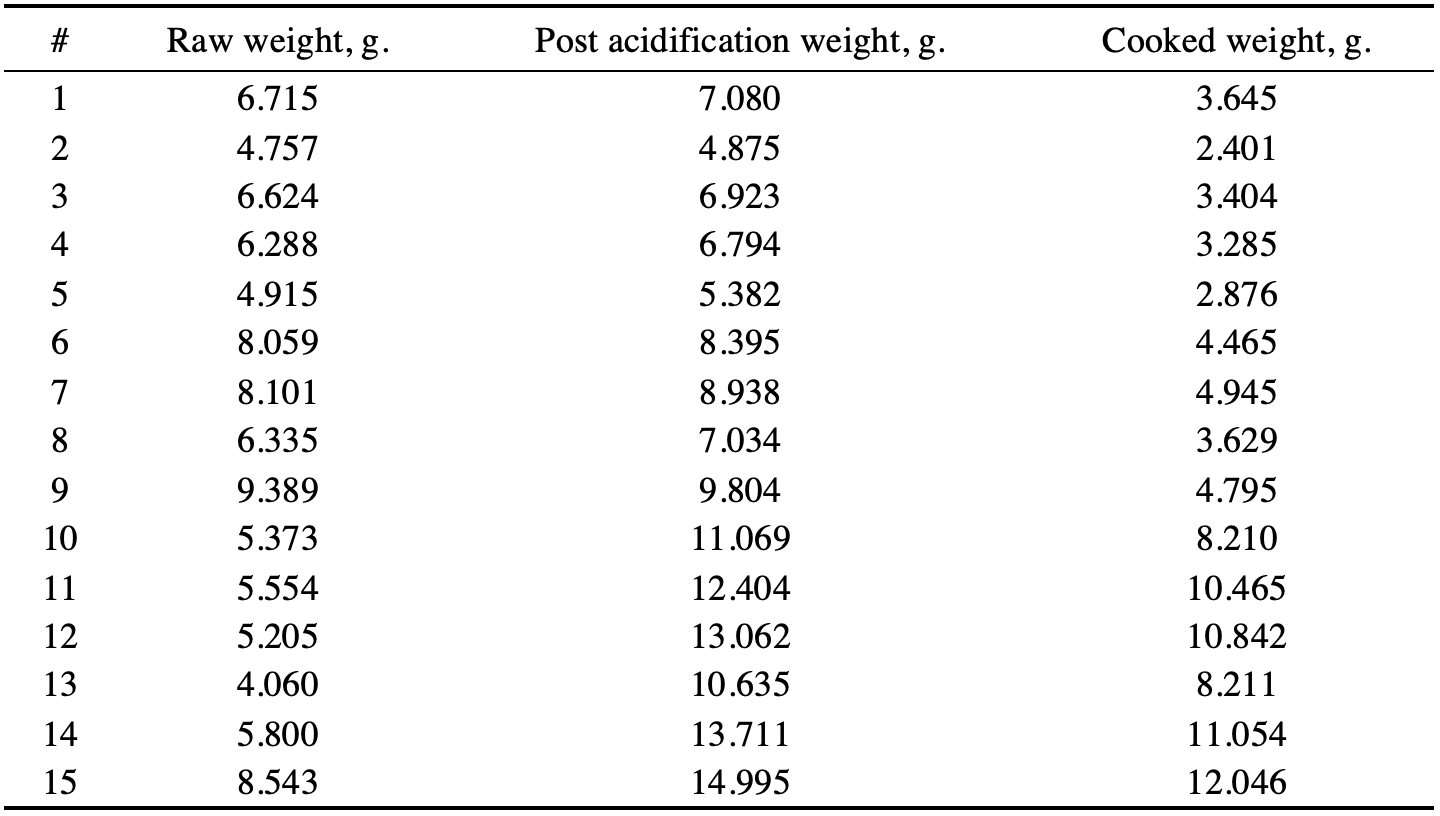
Based on the weights of the chicken samples after and before the return, it was possible to set the water holding capacity factor by dividing the first by the second. For example, for the first sample, WHC was calculated by dividing 7.080 g by 6.715 g for the acid-treated disc and 3.645 g by 6.715 g for the water-cooked disc. Table 2 shows the calculated values for each of the fifteen meat discs.
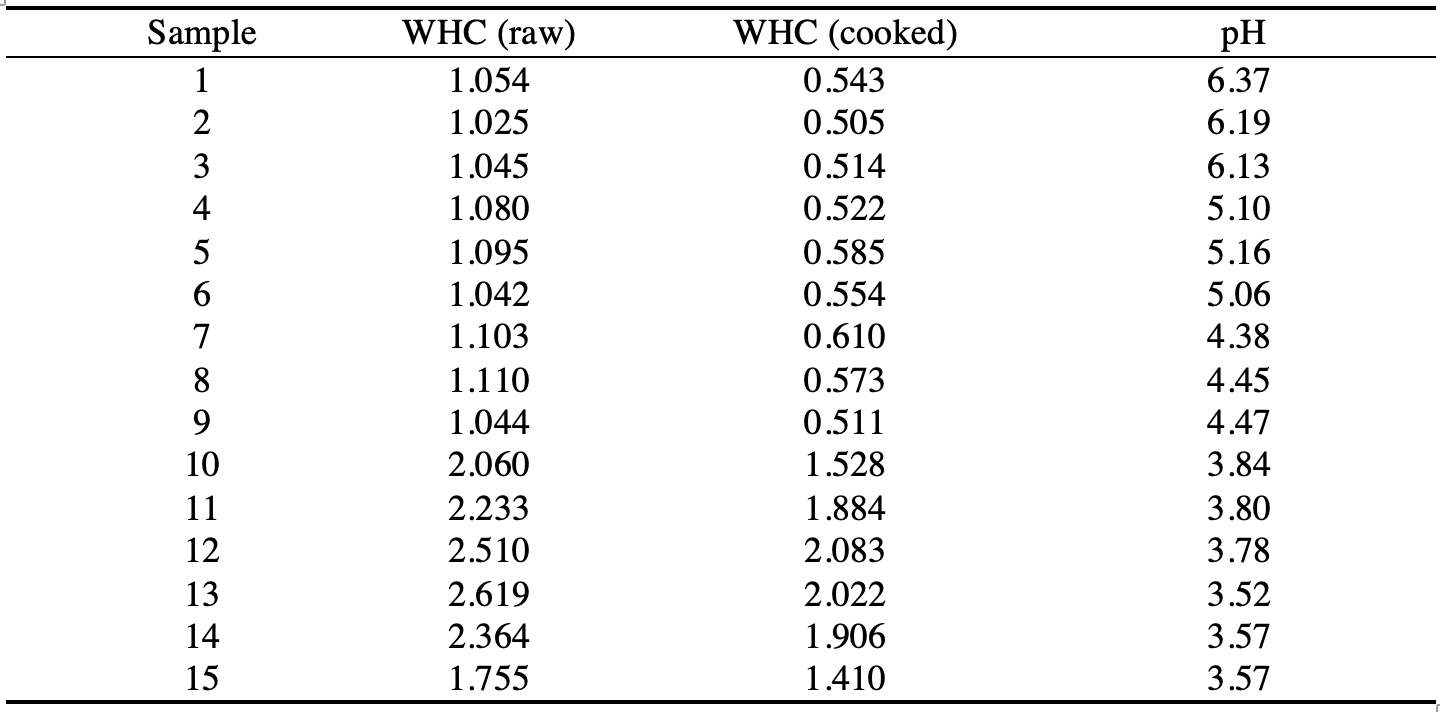
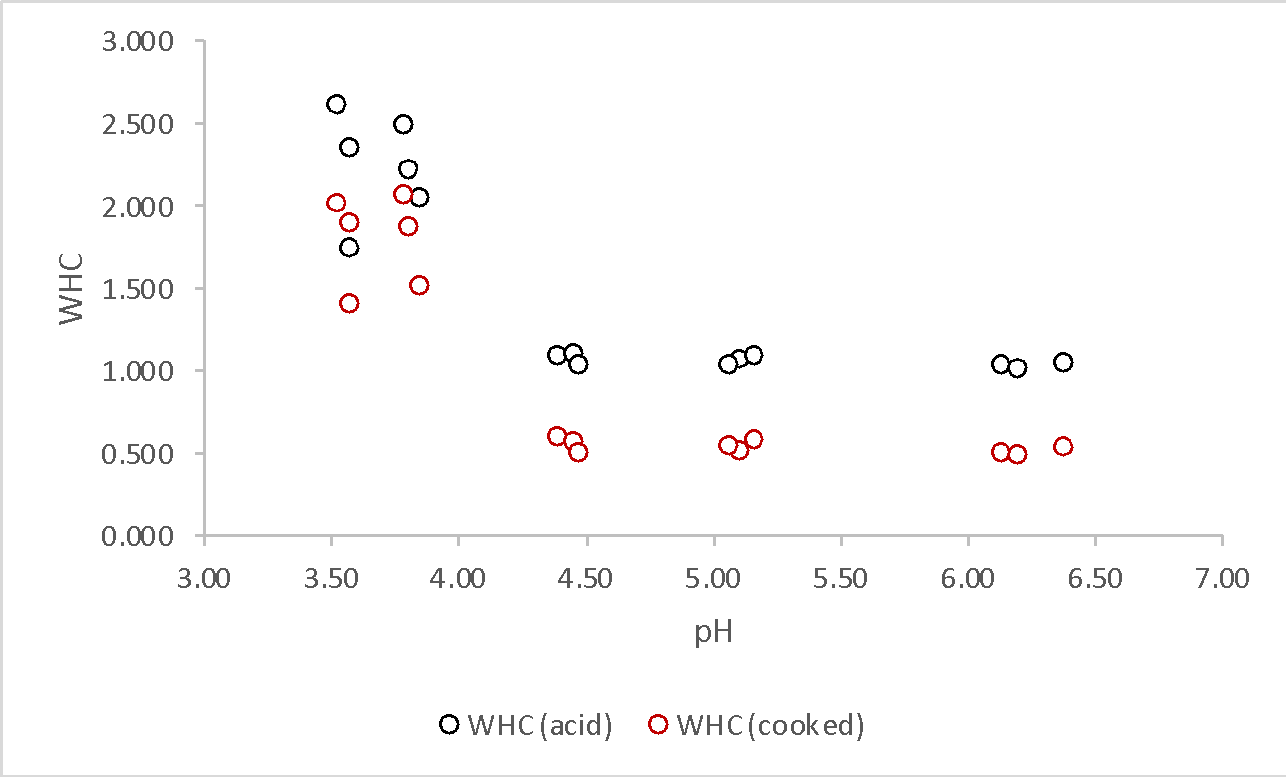
The graph of the pH dependence of WHC for raw and cooked meat was plotted based on the available data. Figure 1 shows a graph of these relationships. At first glance, it appears that an increase in pH is inversely related to WHC since the coefficient values decrease as the acidity of the medium increases. On the other hand, the relationship is indeed not linear because it is seen that the decrease in WHC does not occur uniformly as the pH decreases.
Discussion
This laboratory work aimed to identify the pH dependencies on WHC. The pH was measured once for each sample, in the intermediate period between the acid treatment and the water bath cooking, using a pH meter whose probe tip was placed in the center of the disk. The values obtained determined that the relationship between WHC and pH was not linear and showed a downward trend. This meant that as the acidic environment increased, the WHC decreased.
At first glance, the results seem to contradict theoretical expectations. Academic discourse reports that as pH increases, WHC values should also increase (Khidhir, 2019). However, this is true for high pH values, whereas for low values, the WHC does fall as the acidity of the medium increases (Jankowiak, Cebulska, and Bocian, 2021). Thus, the overall dependence of WHC on pH is inverted bell-shaped. The main reason for this pattern is the isoelectric point, a certain physical and chemical state of the system in which the number of positive and negative charged particles is identical; in this case, a minimum WHC is observed for meat (QPC, 2020). In the case of low pH values, however, there are more positive particles (protons from the acid) in the medium, resulting in bipolar proteins turning into positively charged protein particles with NH3+ ends. The surplus content of positively charged proteins binds water molecules through the negatively charged O-end of the molecule, which contributes to removing water from the meat composition. In other words, the liquid medium absorbs excess water, causing the WHC to drop. WHC values for raw meat were higher on average than for cooked meat, as follows from Figure 1. A likely reason for this could be the cooking process, which causes the chicken proteins to denature and are no longer able to retain moisture in the same way that the non-denatured molecules did.
WHC significantly affects meat because it determines the consumer properties of the product. Maximizing moisture retention in the meat structure allows for the product’s attractive traits, including texture and appearance. At the isoelectric point, the WHC value turns out to be minimal. In this case, the proteins are bipolar, which eliminates excessive water retention or expulsion, as observed at higher or lower pH values. To improve the work, it is possible to investigate the effect of acid concentrations and the nature of these acids on the WHC, which will deepen the knowledge of the topic.
Conclusion
To summarize the present laboratory work, the effect of pH on WHC for raw and cooked meat was evaluated. It was determined that the WHC decreases with increasing pH values, which is true for low acidity levels (3.57 to 6.37 in this work). This meant that in areas of low acidity, excessive amounts of positively charged protein particles caused water to be pushed out of the meat structure, negatively affecting consumer appeal parameters. In this context, it is evident that increasing pH (in the region up to the isoelectric point) negatively affects product quality and is not a desirable measure.
Reference List
Jankowiak, H., Cebulska, A. and Bocian, M. (2021) ‘The relationship between acidification (pH) and meat quality traits of polish white breed pigs’, European Food Research and Technology, 247(11), pp. 2813-2820.
Khidhir, Z. K. (2019) Water holding capacity in meat and color with changes in pH.
QPC (2020) Graphic representation of WHC and important concepts.