Abstract
Fishes possess normal differences that range from gill numbers, arches, and length of the filaments. The most observable is the differences in overall length in fish sizes than determining the gill length by a microscope while held on the stage and measurements are taken directly using vernier calipers. Studies that have been conducted led to the observation of a spectacular difference that the number of red muscles was directly proportional to the length of the fishes.
After a series of research was carried out on different fishes which included various marine fishes, Researchers were amazed at the fact that one of the fishes had the inverse of the proposition that had been stated. Tuna had the contrary with a decrease in the amount of the red oxidizing muscles. This has now been stated to be a normal endowment possessed by the Tuna fish. It is therefore the purpose of this paper to establish the normality of the Tuna’s diminished amount of red muscle as opposed to the increasing amount observed in many fishes.
Introduction
It is quite notable that active fishes have a very big proportion of red muscles in their trunk musculature than the less active fishes possess. Red muscles have further been a tool behind the nomenclature of certain fishes into functional groups reflecting different adaptabilities. Different measures of the red muscles and mobile characteristics have several limitations. However, the relationship between the two has not been proved quantitatively. ”Secondary data strongly support the idea of a very strong association between the proportion of red meat and the mobility” (Sfakiotakis et al, 1999. p 239).
The most essential muscle in this line is the caudal peduncle of marine fishes. Fish has therefore been categorized as sedentary and mobile under the natural history as the school of thought. Further criticism has targeted the frequency of distribution of the amount of red muscle that provides evidence for two sub-divisions, which do not in any way confer association with the sedentary against the mobile class dichotomies. In this context, the ecophysiology of the fish in the marine environment includes Temperature, pH, and salinity, while aerobic performances involve activities that demand the use of oxygen while in marine water.
Materials and Methods
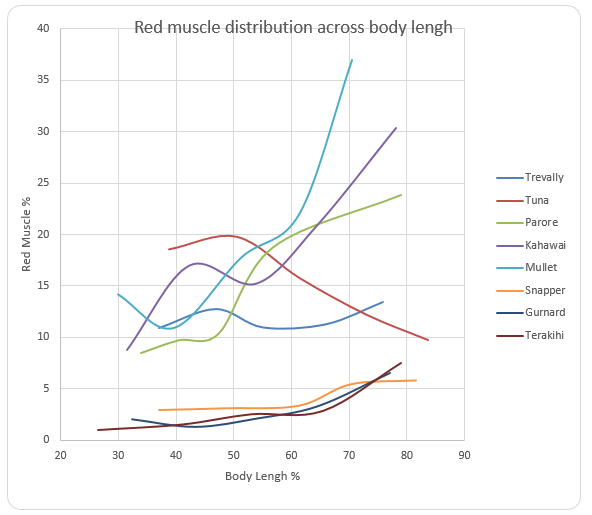
Fish samples were captured, their body length determined, and converted to percentages. The weights of the fishes were also taken. They were then dissected and a fillet of red muscle was removed from both sides of the fish starting from the caudal peducle to the head, it was also removed from the anterior wedge posterior and anterior locations. The weight of the red muscles extracted was determined and converted to a percentage over the whole weight of the body. The data was extrapolated on the graph using an excel spreadsheet and the following observations were recorded.
Results
The red muscle distribution of different parts of the fish generally increased when the body length increase as well with more red muscles for locomotion at the tail and fin. However, only Tuna has a different trend which has been stated to be exceptional and normal for the Tuna fish. It is as shown below.
Discussion
The distribution of lactate oxidase enzymes has been studied in red muscles and results indicated a dominancy of isoenzymes or a selection of which the anodic form dominated. Pyruvate dehydrogenase enzymes also studied did not establish any characteristic that could be used to establish the differences. Lactate oxidase provides strong evidence that fish should be capable of oxidizing the lactate produced by the white muscles during strenuous exercise.
They also possess numerous correlated anatomical specializations that are unique in swimming mechanisms. The question of whether heterothermy is a design or an adaptive feature is still being contested. On this fact, there is a universal expectation that heterothermic fish will perform better than nonheterothermic fish due to the temperature variation. On this Basis, heterothermic is said to be adaptive and the temperature has a direct influence on enzymatic catalysis of reactions, and lactate oxidase is not excluded from this category of enzymatic reactions. The anatomical aspect can also not be exempted from this line of thought as a principle that enables excretion of wastes at a very fast rate, eliminating stress due to accumulation of waste products and by-products while supplying oxygen again to the tissues at a faster rate together with the metabolic fuel.
“The two blood vessels transport adequate oxygen to the powerful oxidizing muscles at any moment during strenuous activity” (Gray, 1996. p 222). Marginal oxidizing muscles are supplied blood by the large artery and the vein pairs. Small blood vessels stem from the main vessels and infiltrate between the surface red and the large bodies of white glycolytic muscles. Small blood channels then divide into smooth vessels that nourish the capillary beds contained by the internal tenderloins of the red muscles.
” The red oxidizing muscle is just a small segment in a position close to the mid-lateral line. This has numerous mechanical consequences; any force generated within any myotome has several trajectories” (Syme and Shardwick, 2001. p 195). Each myotome is separated by a layer of collagen that is a sheet-like called a myoseptum that forms the origin. The red muscle fiber is used for a long duration, low-intensity activity and is located along the mid-lateral line under the skin. “Contrary to the glycolytic also referred to them as a white muscle which is used for high intensity sprinting covers the rest of the body muscles” (Rosenberger, 2001. p 386).
Tuna is one of the fishes that swim at very high speed has more white muscles and fewer red fibers and this can be attributed to the high rate of production of the lactate that is produced and requires the red muscles that have high amounts of lactate oxidase to break down the lactate, Coupled with the very powerful cardiovascular system. The internal tenderloin of red muscles fibers is positioned inside the fillet, medial to the skin, and on the side of the spinal column.
The swimming muscles of Tunas are penetrated by an array of complicated blood vessels that make up the circulatory system. They have the role of supplying the oxidizing muscles with aerated and nutrient-rich blood as well as transduction of heat or limiting the conduction of heat to other parts of the body. Tuna has a special type of gigantic scale structure of the circulatory system of the thermogenic red muscle. The most interior red muscle is fed by large pairs of blood vessels positioned alongside either side of the body from the middle hemal that bears the aorta and the cardinal vein.
Temperature, pH, and Oxygen influence the oxygen-binding capacities in the marine aquatic environment that constitutes the energetics of the body cells. The most unique feature of the red fibers is the hemoglobin, but now in the muscles are referred to as myoglobin. They functionally transport oxygen to tissues and cells enabling catabolic reactions to take place within the body for the reaction that leads to the generation of ATP, which is a kind of energy that is utilized by body cells. The physiological adaptability to temperature-dependent distribution has the effect of extracellular Mg 2+ and k+ ion regulation leading to exploitation of the antagonistic effects of the Magnesium, Potassium, and calcium ion channels.
The biochemical basis for maintained firm swimming and burst swimming depends on different metabolic reactions that generate ATP which is produced by the contraction of muscles. These are aerobic pathways of ATP production that burn down useful energy sources within the muscles. “Enzymes such as creatinine phosphate provide ATP through the direct phosphorylation of ADP” (Gordon, Chin, and Vojkovich, 1989. p 352). Similarly, the enzymatic effect on the glycogen stores of the glycolytic pathway and lactate dehydrogenase lead to the production of lactate and NAD to maintain the redox reaction during aerobic glycolysis.
Summary
It can therefore be deduced that burst swimming in aerobic performance could be enhanced by increased amounts of lipophilic fuels; dominancy of lactate dehydrogenase and glycolytic enzymes; high-level buffering capacity for the muscles; raised myosin ATPase activity, Ca++ATPase activity that is coupled to K+ ionic activities, and/or parvalbumin concentration whose function is activated transport of all in the white myotomal muscle cells.
On the other hand, swimming can be sustained with increased oxygen supply to the red oxidizing muscles which are influenced by the well structured circulatory system; increased metabolism of aerobic energy sources that lead to the generation of ATP by the red muscle; well loaded lipogenic stores whose catabolism demand a more aerated environment in the red muscle to produce energy; a high number of mitochondria that are contained on the red muscle for effective oxidative phosphorylation and thus acting as a powerhouse, or increased aerobic enzymatic reactions of ATP production that rely on catalysis by the presence heat and oxygen.
Reference list
Gordon, S. M. Chin, G. H. Vojkovich, M. (1989). Energetics of swimming in fishes using different methods of locomotion: Labriform swimmers. Fish Physiology and Biochemistry. Amsterdam/ Berkeley: Kugler Publication. Vol. 6. No6.
Gray, I. E. (1996). Comparative study of the gill area of marine fishes. Department of Zoology. Durham, N. C: Duke University.
Rosenberger, L. (2001). Pectoral fin locomotion in batoid fishes: Undulation versus oscillation. Journal of Experimental Biology. Great Britain: Biologists Limited. Vol. 204. No. 12.
Sfakiotakis, M. Lane, M.D. Bruce, J. Davies, B. (1999). Review of swimming Modes For Aquatic Locomotion. IEEE journal of oceanic Engineering. Vol. 24. No. 2.
Syme, A. D. Shardwick, E. R. (2001). The effect of longitudinal body position and swimming speed on mechanical power of deep red muscle from skipjack tuna. (Katsuwonus Pelamis). The journal of experimental Biology. Great Britain: Biological limited. Vol. 205.