Introduction
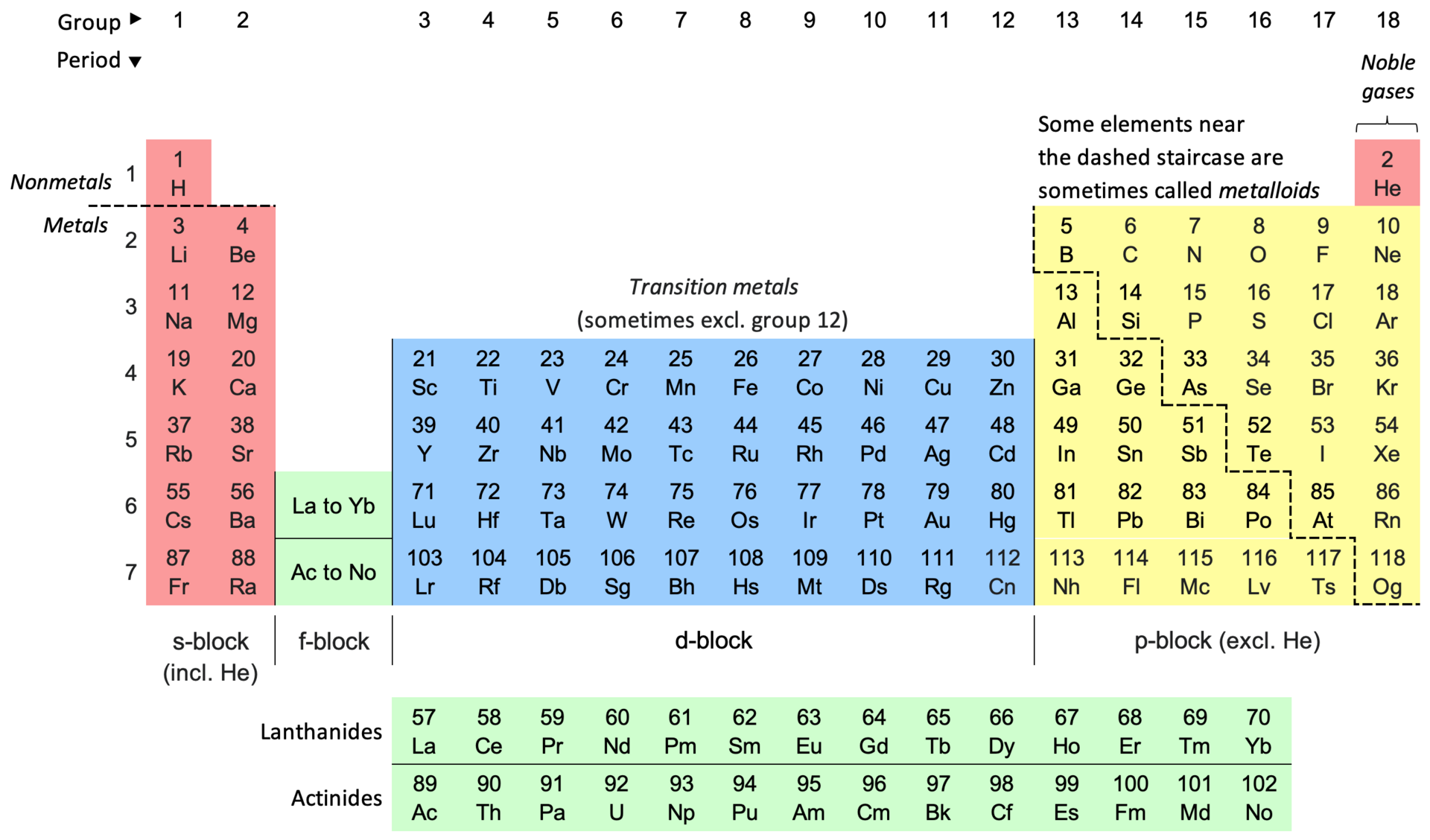
The periodic table of elements is a central tool widely used by chemists and physics due to its remarkable classificatory and predictive properties. The table arranges the chemical elements into rows (periods) and columns (groups) based on their atomic structure and properties (see Figure 1) (Kumar and Bhargav, 2022). This paper will explain this arrangement’s functionality and explore the table’s main analytical applications, including the importance of the periodic table’s s, p, and d blocks.
Main Body
The periodic table is divided into 18 main groups, often labeled from 1 to 18. These groups share similar chemical properties due to their identical valence electron configurations. For example, Group 1 elements (alkali metals) all have one valence electron, making them highly reactive. In contrast, Group 18 (noble gases) elements have full valence electron shells, rendering them chemically inert.
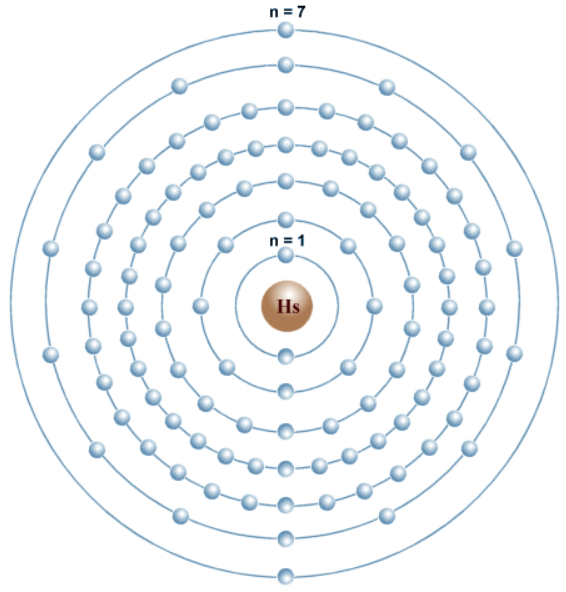
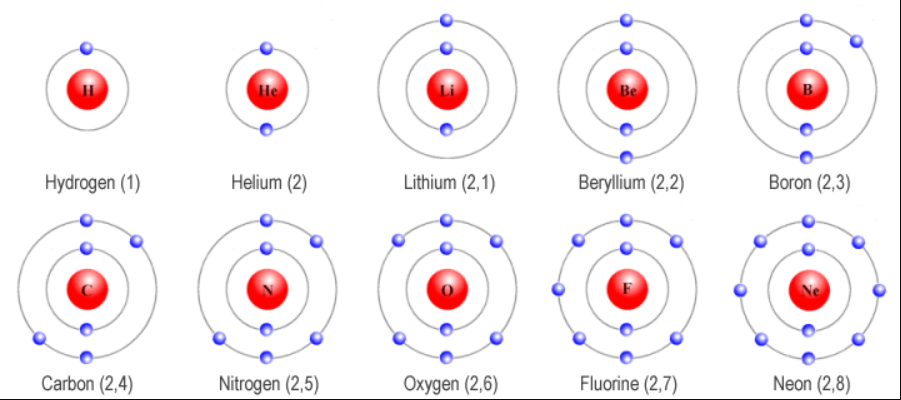
The numbering of the periods (rows) indicates the number of electron shells an element’s atoms possess. In the Rutherford-Bohr model of the atom, electrons occupy specific electron shells with corresponding energy levels represented by a quantum number (n), which ranges from 1 to 7, from smallest to highest (see Figure 2) (Kumar and Bhargav, 2022). Each period corresponds to a new energy level, and as one moves from left to right across a period, the atomic number increases by one. For instance, elements of period 3 have three electron shells, and their properties reflect the filling of these shells. Based on the known electron configurations for the first 108 elements, it is possible to predict the orbital properties of heavier elements with a certain degree of certainty (see Figure 3).
Significance of the s, p, and d Blocks
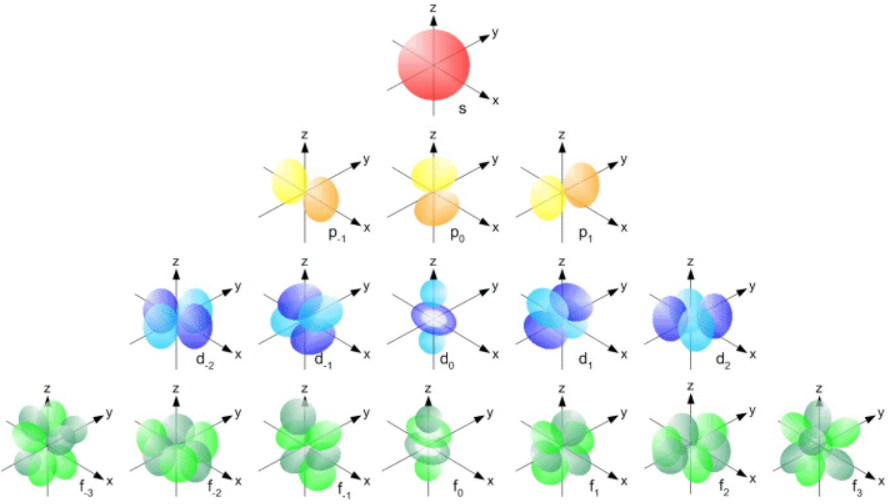
The periodic table can be divided into four blocks: s, p, d, and f, with the first three being of the highest practical interest (see Figure 4). There is a loose correlation between this categorization and sets of elements based on their chemical properties, which makes these blocks helpful for understanding elements’ electronic structure and properties.
- S-block: Found on the left side of the periodic table (Groups 1 and 2), the s-block elements have their outermost electrons in s orbitals. These elements are known for their high reactivity due to their tendency to lose these outer electrons to achieve a stable electron configuration.
- P-block: Located in the middle section of the periodic table (Groups 13-18), the p-block elements have their outermost electrons in p orbitals. They exhibit diverse chemical properties and can form covalent bonds by sharing electrons.
- D-block: The d-block comprises the transition metals between the s and p blocks. These elements have partially filled d orbitals and exhibit many oxidation states and complex formation abilities.
Trends Across Periods and Predicting Properties
Trends in Period 3 of the Periodic Table
In period 3 (comprising elements from sodium to argon), several trends are observed:
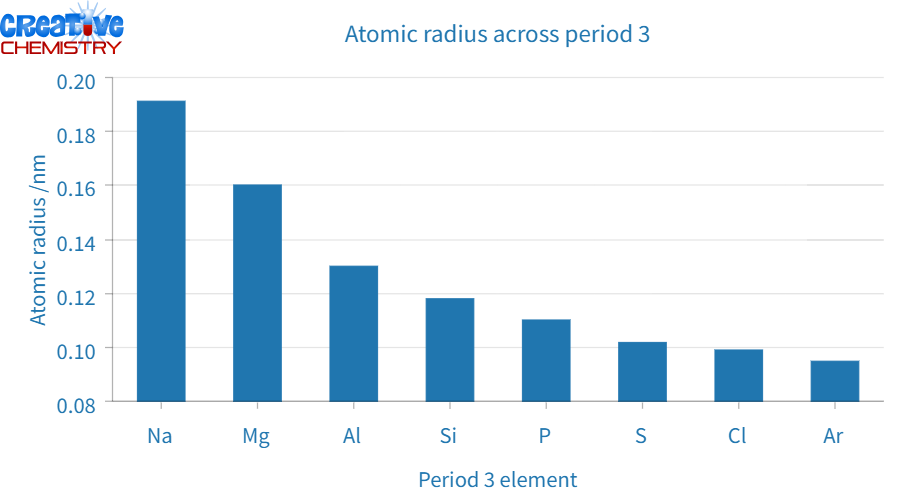
Atomic radius decreases
As one moves from left to right, the increasing positive charge in the nucleus attracts electrons more strongly, resulting in a smaller atomic radius (see Figure 5). This makes the period 3 elements increasingly less reactive to a certain point due to increasingly strong electron-nucleus bonds inside the atom.
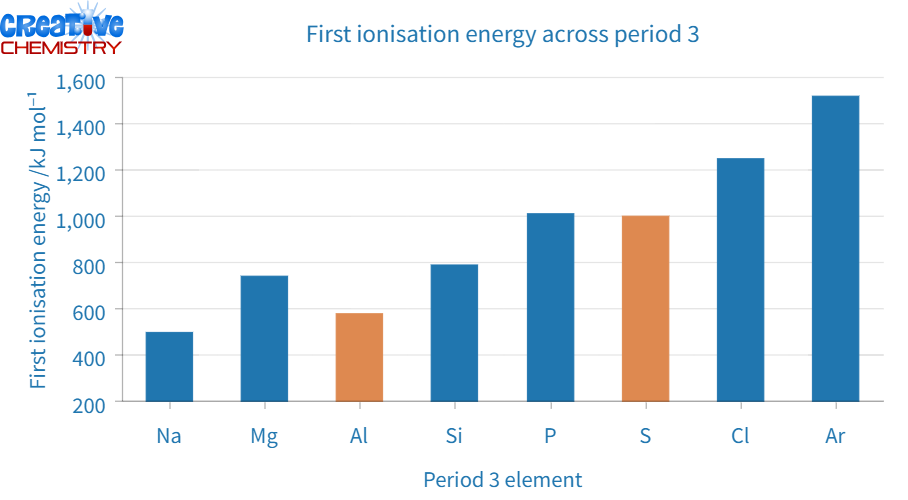
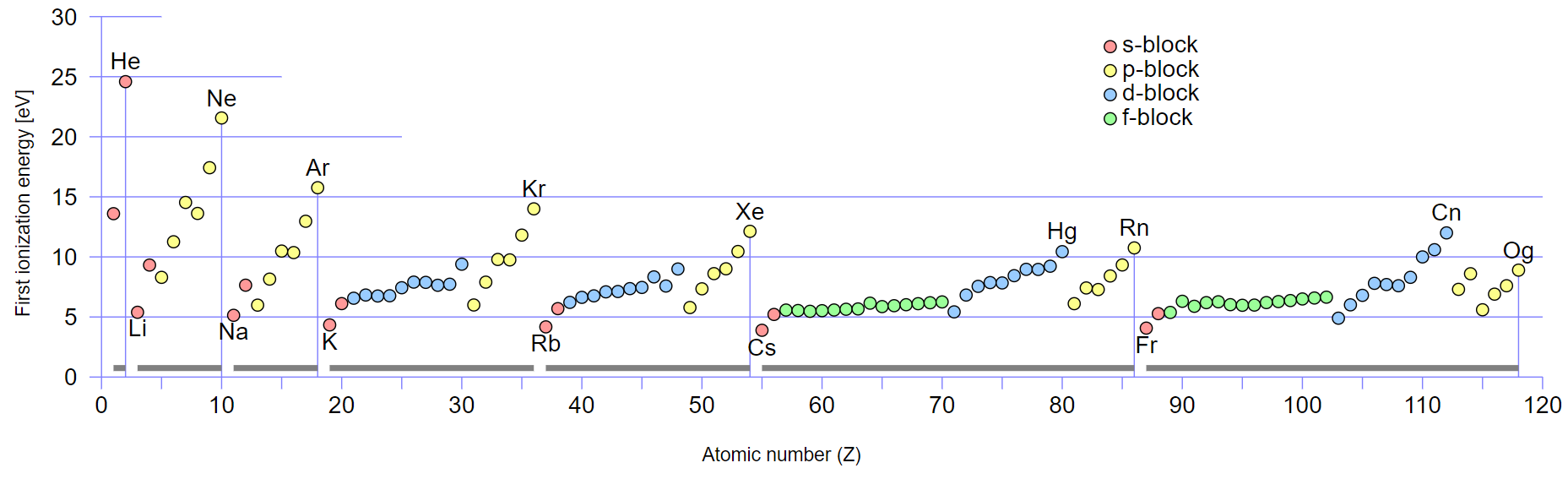
Ionization energy increases
With a smaller atomic radius, it becomes more challenging to remove an electron, leading to higher ionization energies (see Figure 6). Due to an increased number of protons in each nucleus, its nuclear charge increases, thus increasing the force of attraction between the nucleus and outer electrons, with each successive electron entering the same shell. However, there are notable drops in ionization energy for aluminum and sulfur. Looking at the elements’ electronic configurations reveals that the outer electron in aluminum is in a p sub-shell:
- Magnesium: 1s² 2s² 2p⁶ 3s²
- Aluminium: 1s² 2s² 2p⁶ 3s² 3p¹
Since aluminum’s outer electrons have higher energy than magnesium’s, less energy is required to remove them. For phosphorus and sulfur, the situation is different:
- Phosphorus: 1s² 2s²2p⁶ 3s² 3p³
- Sulfur: 1s² 2s² 2p⁶ 3s² 3p⁴
In this case, the 3p electrons in phosphorus are all unpaired, while two in sulfur are paired. The repulsion force between these paired electrons in sulfur makes each of them easier to remove, thus reducing the element’s first ionization energy level.
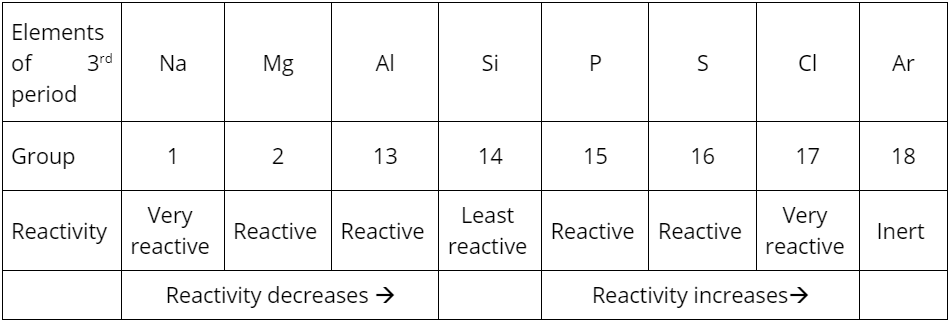
Electronegativity increases
Elements become more electronegative as they gain electrons to achieve a stable electron configuration. As a result, the elements’ reactivity gradually decreases up to group 13 and then starts increasing (see Table 1).
Using the Periodic Table to Predict Properties
By understanding these trends, it is possible to predict the properties of elements in Period 3:
- Sodium (Na) is highly reactive due to its low ionization energy; it readily forms sodium ions (Na+).
- Silicon (Si) shares characteristics of both metals and non-metals, forms covalent compounds, and has a high melting point.
- Phosphorus (P) can form covalent compounds with other non-metals and exists in various allotropes, such as white phosphorus and red phosphorus.
- Chlorine (Cl) has a high electronegativity and readily gains electrons to form chloride ions (Cl-), making it a strong oxidizing agent.
- Argon (Ar), a noble gas, is inert and does not readily participate in chemical reactions due to its full valence electron shell.
- Sulfur (S) forms covalent compounds and, similarly to phosphorus, exists in various allotropes, such as rhombic sulfur and monoclinic sulfur.
Ionization Energy and Electron Configuration
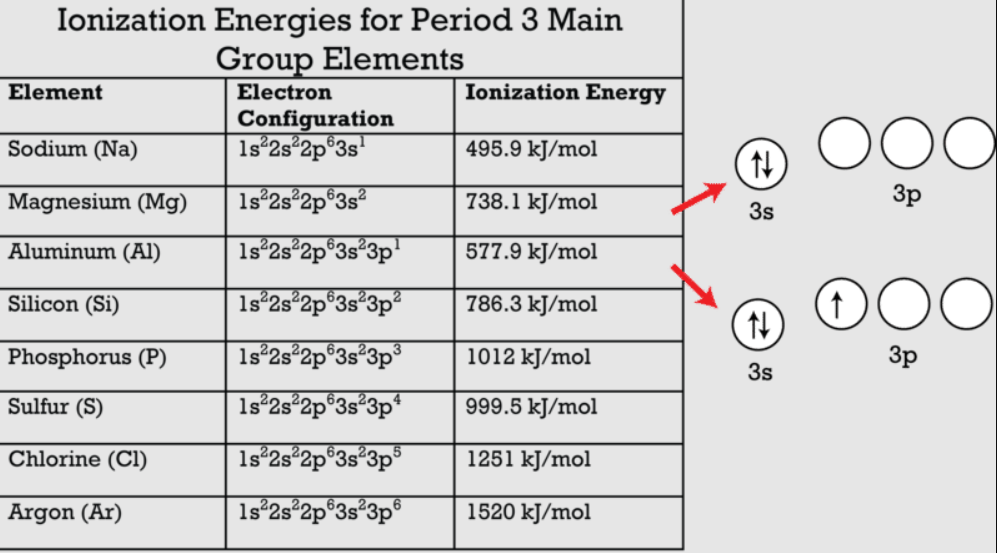
Ionization energy trends can be used to predict electron configurations. This is explained by the correlation between ionisation energy value, atomic radius, and orbitals distribution (see Figure 5, Figure 7, Figure 8). Elements with low ionization energies tend to lose electrons, resulting in noble gas configurations. For example, sodium (Na) has an electron configuration of 1s² 2s² 2p⁶ 3s¹, and it loses one electron to achieve the stable configuration of neon (Ne) with 1s² 2s² 2p⁶.
Trends in Metals within a Group
Reactions of Group 2 Metals with Oxygen
Group 2 metals, such as calcium (Ca) and magnesium (Mg), react vigorously with oxygen to form oxides:
- Calcium oxide (CaO): Ca + O₂ → CaO
- Magnesium oxide (MgO): Mg + O₂ → MgO
These reactions involve the metal atoms oxidation (losing electrons) to form metal cations (Ca²⁺, Mg²⁺) and oxygen atoms gaining electrons to form oxide ions (O²⁻).
Reactions of Group 2 Metals with Hydrochloric Acid
When group 2 metals react with hydrochloric acid (HCl), they undergo oxidation-reduction reactions:
- Calcium (Ca): Ca + 2HCl → CaCl₂ + H₂
- Magnesium (Mg): Mg + 2HCl → MgCl₂ + H₂
In these reactions, the metal atoms are oxidized (lose electrons) to form metal cations, while hydrogen ions (H⁺) are reduced to form hydrogen gas (H₂).
Reactions of Group 2 Metals with Water
Group 2 metals also react with water, producing metal hydroxides and hydrogen gas:
- Calcium (Ca): Ca + 2H₂O → Ca(OH)₂ + H₂
- Magnesium (Mg): Mg + 2H₂O → Mg(OH)₂ + H₂
These reactions illustrate the increasing reactivity of group 2 metals as one moves down the group.
Trends in Non-Metals within a Group
Group 7 Elements (Halogens)
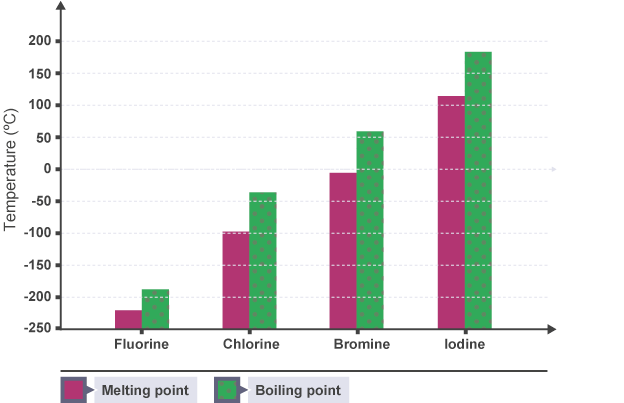
Group 7 includes non-metals, also known as the halogens. This group consists of fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At). Halogens exist as diatomic molecules (F₂, Cl₂, etc.) and display a trend of increasing melting and boiling points down the group (see Figure 9) (Seth, 2020). For example, fluorine and chlorine are gases at room temperature, while bromine is a liquid, iodine is a solid, and astatine is a rare and unstable solid or possibly a gas. The atomic radius also increases due to the addition of more electron shells.
Halogens are highly reactive non-metals known for their ability to gain electrons (reduce) in chemical reactions. This trend can be attributed to the increase in the number of electrons and stronger London dispersion forces between molecules as one moves down the group (Rastogi and Goyal, 2021). As a result, they are known as strong oxidizing agents due to their ability to form a stable halide ion with a full valence shell.
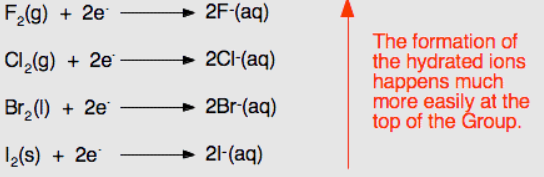
Reactivities and Oxidizing Powers of Group 7 Elements
Reactivity and oxidizing power increase as one moves up the group (see Figure 10). Fluorine is the most reactive halogen and the strongest oxidizing agent, readily accepting electrons in reactions, while iodine is less reactive and exhibits weaker oxidizing properties (Seth, 2020). Hydrogen fluoride (HF) and hydrogen chloride (HCl) react with hydrogen to form hydrogen halides:
- HF + H2 → 2HF
- HCl + H2 → 2HCl
More reactive halogens can displace less reactive ones from their salts. For example, chlorine can displace iodine from potassium iodide:
- Cl2 + 2KI → 2KCl + I2
Conclusion
In summary, the periodic table’s arrangement, including the main groups and blocks, allows for predicting the properties of elements and understanding trends in atomic structure and reactivity. This knowledge is foundational in chemistry and physics and is essential for predicting the behavior of unknown elements or compounds. As a result, it is possible to theoretically model the properties of currently unknown elements and develop detailed methodologies and precise instruments for testing these predictions with high accuracy. Recommendations for the academic context include focusing on interactive possibilities of modern technologies and software to help students gain a more multilayered and detailed understanding of the periodic table’s properties. Those can include 3-dimensional models, digital playground/experiment environments, and virtual reality workspaces allowing students to test various predictions based on the periodic table’s discussed properties.
Reference List
Atomic radius across period 3(no date). Web.
Clark, J. (2022) The formation of the hydrated ions happens much more easily at the top of the group. Web.
Double sharp (2021) Graph of first ionisation energies. Web.
First ionisation energy across period 3 (no date). Web.
Ionization energies for period 3 main group elements (2013). Web.
Kumar, A. and Bhargav, K. (2022) Foundation course in chemistry with case study approach for JEE/NEET/Olympiad class 9. 5th edn. Delhi: Disha Publication.
The melting and boiling points of the first four group 7 elements (no date). Web.
Rastogi, S. C. and Goyal, M. (2021) Chemistry class XI. Agra: SBPD Publications.
Sandbh (2023) Coloured periodic table showing the most common sets of elements. Web.
Seth, C. (2020) Gateway to science — chemistry for class X. Delhi: Goyal Brothers Prakashan.
Trends of periodic properties in periodic table and reason of variation (2023). Web.
Wells, C. J. (no date a) Electron orbitals can have complex geometries. Web.
Wells, C. J. (no date b) The element Hassium (Hs) has 108 electrons in 7 electron shells. Web.
Wells, C. J. (no date c) The first ten elements in the periodic table. Web.