The production of Aluminum is through the Hall-Heroult process that Hall and Heroult invented in 1886. This method entails the dissolution of alumina (Al2O3) into molten cryolite (Na3AlF6) at 1223K or 950 degrees C. The resulting product is oxygen ions and aluminum metal. Given that the density of molten aluminum is higher than that of molten cryolite, the former goes to the bottom of the bath. Oxygen reacts with a carbon anode to produce CO2. The equation for this reaction is: 2Al2O3 (solution) + 3C(s) = 4Al (l) + 3CO2 (g).
While the Hall-Heroult process has been around for about 120 years, the chemistry and physics behind the process started unfolding in recent years with the arrival of numerical methods and computing techniques. Initially, one could carry out mathematical modeling by carrying out some calculations on the sidewall ledge. Later on, things changed and there was a need for one to know the magnetohydrodynamic effects properly.
In addition to the MHD forces, the other force that acts on the bubbles is in the form of buoyancy and it originates from an anode. Further, the bubbles induce certain uniformity between bath’s temperature field and the distribution of the solid alumina. Besides, they increase the energy consumption or ohmic overvoltage of the cell. Moreover, they enhance a stable operation as they perturb the bath-metal’s interface and also by provoking the anode effects. A balance between the inputs and outputs should be in place. The inputs are energy, charge, carbon, or alumina, whilst the possible outputs may include gases evolved, heat generated, and aluminum in its liquid form. The solid alumina is vertical, introduced in the electrolyte (bath) either between the anodes or in the sidewall’s channels. The alumina should be moved and distributed horizontally within the inter-electrode space. Gravity does not only separate the aluminium metal but also expels any gas generated from the process of electrolysis.
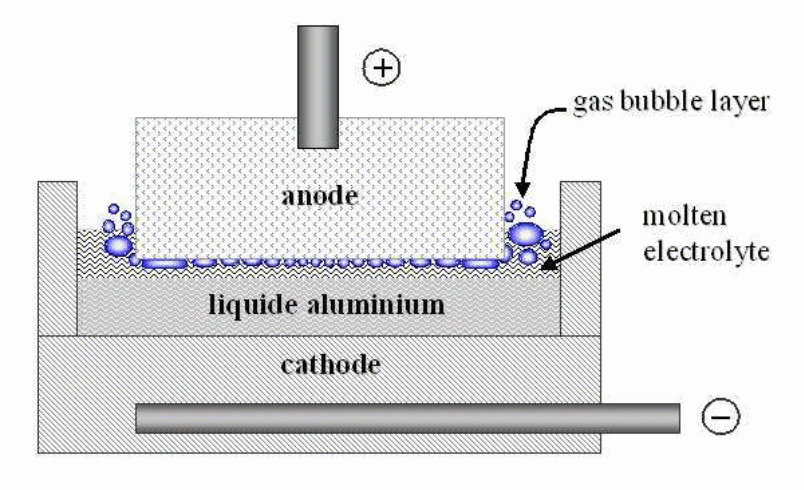
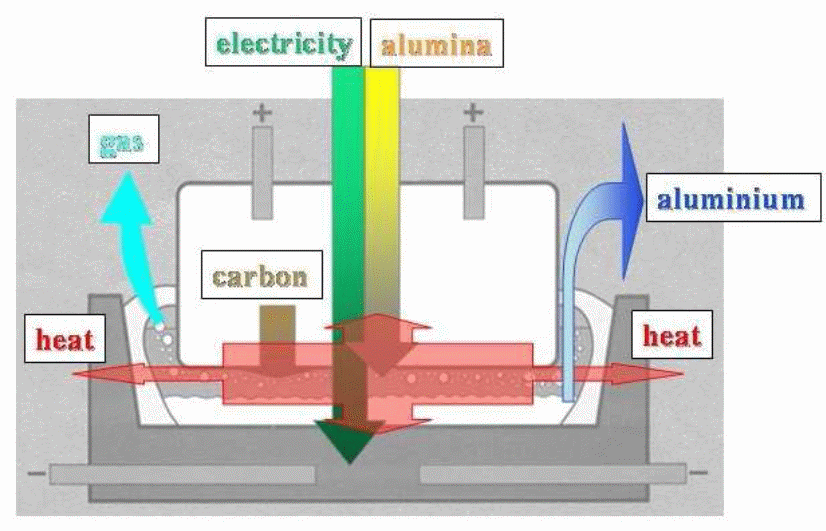
This electrochemical reactor comes with a unique geometrical dimension: there is a vertical movement of aluminum and the electric charge travel whilst the horizontal motion entails the movement of different gases, the solid alumina plus heat. To achieve an optimal reaction in the system, energy and mass should move in a horizontal pattern achieved through the homogenization of temperature and concentration–intensive components. The bubbles and MHD effects enhance the horizontal motion in the cell.
As the reaction proceeds, the bubbles (just like a motor) enhance the change from gravitational to kinetic energy and all this takes place in a horizontal kind of flow.
The processes within the cell depend mainly on convection that takes place in the cell’s horizontal section. This means that vertical convection may not have a place in the process. When there is vertical motion occurring as interface waves or disturbance in the outer layer of the bath, a negative outcome may occur, which may include the reoxidation of aluminum, various electric shortcuts and other effects. A great challenge in the cell design is to minimize vertical movement and promote mixing within the horizontal plane.
The anode area emits a gas that creates a thin, bubble layer measuring 4-5 mm at a minimum and about 10-12 mm amidst dynamic conditions. Either the elongate “gas-hold up” or bubble coalescence causes enlarge the bubble to become “Fortin Bubbles”. Gas hold-up results from an equal release and expulsion of gas within a given time. This causes a considerable variation in the amount of gas that collects near the anode. The bubble’s shape is affected by gravitational and drag forces, surface tension, and contact angle. Studies show that the bubble undergoes a sliding mechanism through a creeping motion that separates the bubble from the solid region. Besides, it is observed that the bubble’s shape evolves from spherical to ellipsoidal and finally to the bubble-cap shape based on the size and velocity of the bubble. It is also observed that during sliding, there is a bubble wake that may lead to localized phenomena.
In 2010, the bubbles were created from air injection and the results show that the symmetrical shape of the bubble is lost after detaching from the sidewall. Generally, the velocity of the bubble increases at the point of proximity with the wall when the surrounding fluid is highly viscous and due to the sidewall’s effect. On the contrary, the bubble’s velocity reduces in a lower viscous fluid. The difference results from the change in shape and the asymmetric flow near the bubble. Also, the bubble tends to stretch as the width of the side channel reduces. The analysis of bubble formation is applied in an aluminum reduction in which the CO2 formed at the anode influences the flow field that exists between the two electrodes. Specifically, this gas restricts the possible flow of current, causes a high resistance and eventually increases energy consumed during the process. The anode effect represents a good case in which the gas totally blocks the anode’s base.
A major challenge when it comes to modeling transport phenomena during electrolysis has to do with the varying scales of time and length. A flow that emanates from the bubbles occurs on a one-meter scale pattern even though bubble section is less thick. The millimeter-scale can help one to understand the different ways that involve the momentum force around the bubbles.
Recent studies show that during early growth, the rate of change in the bubble’s thickness and aspect ratio indicated an agreement between the predicted and experimental results using the phase-field method. Besides, the numerical method gave a prediction of the transient pressure revealing the change from formation to enlargement regime in which there is pressure change along with the interface from the positive to negative value. Also, at the initial growth, the rate of gas injection, the orifice diameter, and surface tension influenced the bubble shape. A change in shape at the initial condition creates a larger, radial displacement than a base displacement of a bigger bubble with a flattened top, which may cause a localized flow in the Hall-Heroult cell. The beginning of the change from the spherical to the non-spherical shape occurred faster for the fluid with lower surface tension. The lifecycle of the bubble within an electrolyte is marked with different periods and sudden events separate one period from the other one. The events may include the beginning point of a growth process, bombardment with similar bubbles, and their detachment. Nevertheless, this observation requires further investigation verification of the other systems if the findings have to be credible.