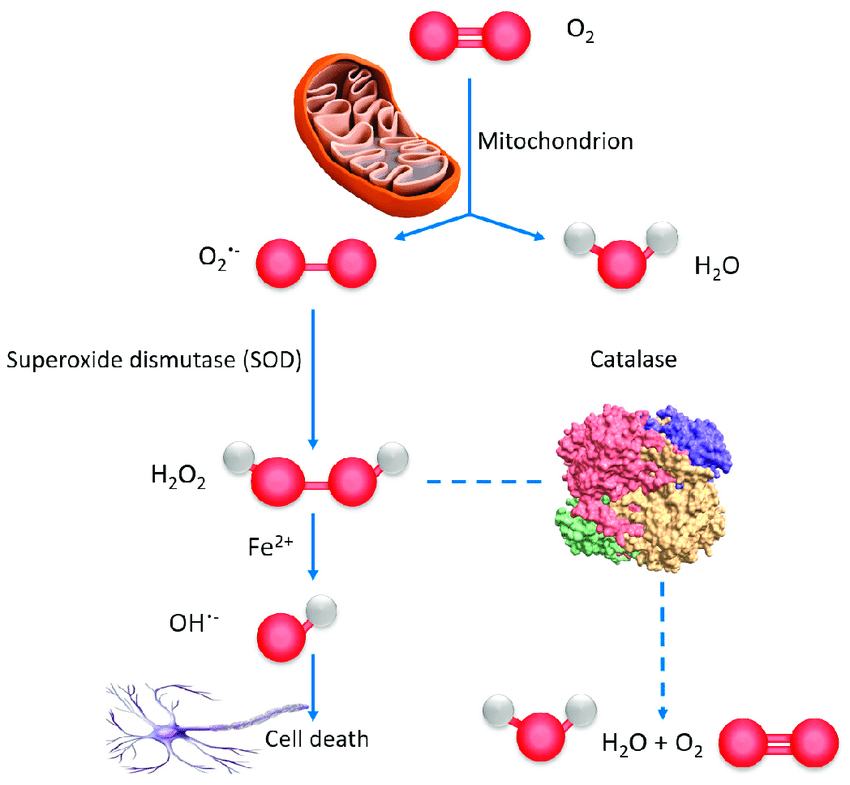
The main reactive oxygen species include the superoxidanion radical (O2-˙), hydroxyl radical (OH˙), hydrogen peroxide (H2O2), singlet oxygen (‘O2) and others. Both under pathological and physiological conditions, the formation of ROS occurs in several biological systems, such as the respiratory chain of mitochondria, the electron transport chain of microsomes, during the transition of oxyhemoglobin to methemoglobin, in the metabolism of arachidonic acid, in the hypoxanthine-xanthine oxidase reaction, during biosynthesis and oxidation of catecholamines, etc. In the case of mitochondria, this is explained by the fact that in the respiratory chain there is a “leakage” of electrons from I and III mitochondrial enzyme complexes, due to which about 2–5% of the incoming oxygen passes into the active form, while part of the ROS goes for oxidative modification of macromolecules. The production of O2-˙ in mitochondria is carried out in several ways and significantly depends on the activity of respiration and changes in the partial tension of oxygen, hypoxia or reoxygenation. The main site of electron leakage from the respiratory chain and, consequently, the formation of O2-˙ is ubiquinol cytochrome C oxidoreductase, where ROS are generated due to one-electron reduction of molecular oxygen from ubisemiquinone. With a change in the intensity of the electron flow and the degree of reduction of the components of the mitochondrial respiratory chain, the number of outgoing electrons also changes. In this case, in the case of the reaction of the formation of a hydroxyl radical, active iron is released, as can be seen in Figure 1.
However, increased ROS production leads to oxidative stress. From a chemical point of view, oxidative stress is a significant increase in the cellular redox potential or a significant decrease in the reducing ability of cellular redox pairs, such as oxidized/reduced glutathione. The effect of oxidative stress depends on the severity of its severity. Cells can return to their original state with minor disturbances. Depending on the strength of stress, cells can die as a result of apoptosis, when the internal contents of the cell have time to degrade to non-toxic decay products, or as a result of necrosis, when the strength of oxidative stress is too great. In necrosis, the cell membrane is disrupted and the contents of the cell are released into the environment, which can result in damage to surrounding cells and tissues.
Reference
Rakotoarisoa, M., Angelov, B., Espinoza, S., Khakurel, K., Bizien, T., & Angelova, A. (2019). Cubic liquid crystalline nanostructures involving catalase and curcumin: BioSAXS study and catalase peroxidatic function after cubosomal nanoparticle treatment of differentiated SH-SY5Y cells. Molecules, 24(17), 3058. Web.