Experimental Procedures
The performance of the present laboratory work is based on an experiment whose purpose is to synthesize aspirin as an analgesic agent from salicylic acid. To obtain the substance, 138 mg (exactly) of salicylic acid, one drop (2-3 mL) of 85% orthophosphoric acid solution, and 150 µL of acetyl anhydride were placed in a glass reaction tube — the reaction proceeding was exothermic, and proceeded with abundant heat release. A boiling stone was added to the reaction tube to create a uniform synthesis and a calmer process. The test tube was heated at 90 °C for five minutes until all solids were dissolved, thus forming a homogeneous solution. The excessive amount of anhydride was neutralized by the addition of 0.5 mL of water, after which the solution was cooled in a water bath until the aspirin crystallized. The solid residue was filtered in a Hirsch vacuum funnel, and the impurity residue was removed by thorough washing with distilled water. The resulting product (aspirin) was dried and further used for laboratory analytical procedures, namely accurate weighing and IR spectra.
Results
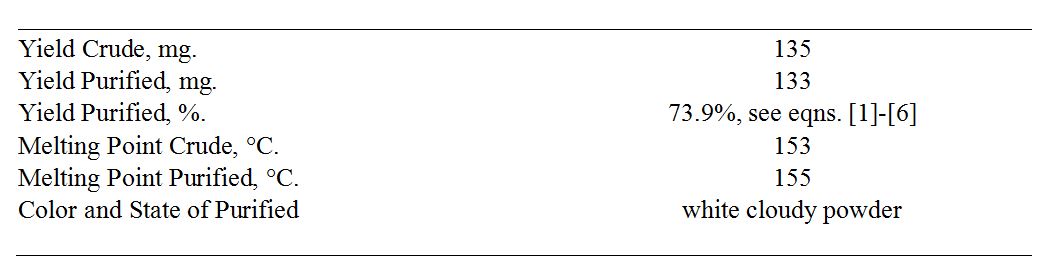
The results of direct measurements and calculations, including calculations of the percentage yield, are shown in Table 1 and equations [1]-[6].
Table 1. Results and outcomes of the experiment
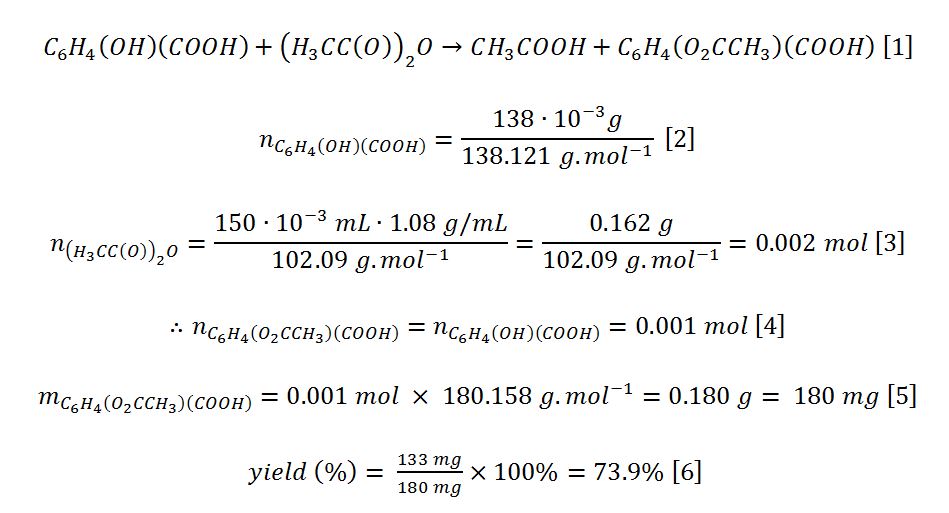
IR spectrographic analysis was performed for the obtained substance (aspirin), the result of which is shown in Fig. 1. The absorbance of the characteristic peaks (Table 2) of the substance differs from the reference, which may indicate the presence of impurity compounds (NIST, 2021). The presence of impurities was also confirmed by the difference in melting point, as the reference melting point is 135 °C, as well as in the moderate percentage yield (NIST, 2021). In other words, the filtration was imperfect, which resulted in a lower yield.
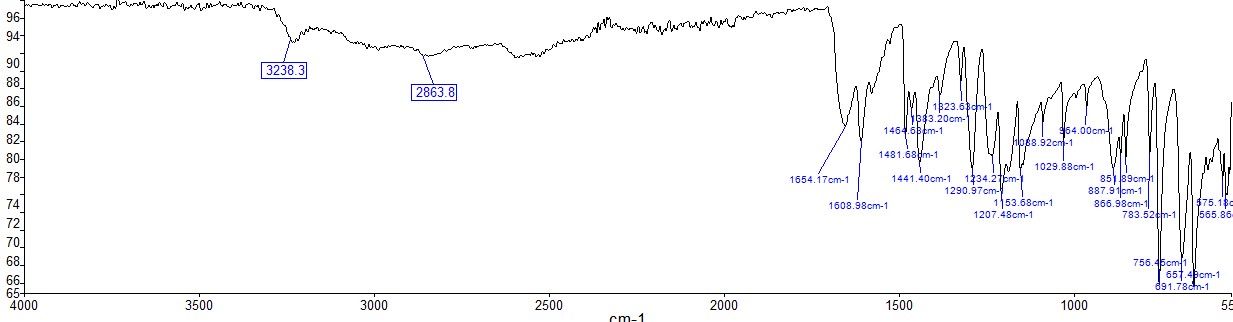
Table 2. Comparison of characteristic peaks for two substances
Reference
NIST. (2021). Aspirin. NIST Chemistry WebBook, SRD 69. Web.