Introduction
The present work was concerned with the empirical determination of acetic acid concentration in commercially traded commodities by means of a titration procedure. For identification, a NaOH solution was standardized, which was then used for acetic acid titration.
Method
Standardization is the process of quantifying the exact concentration of a solution using a titration procedure (IU). In the laboratory, and especially for analytical purposes where the concentration of a substance is of high importance, it is not easy to trust the values on labels, so titration can be used to determine the concentration precisely. In a titration, a substance with a known concentration is added to a substance with an unknown concentration, and the volume used is used for calculations.
Titration was based on acid-base interactions in which an acid donates a proton to the base, resulting in the formation of a salt and water. During this procedure, acid-base interaction indicators that indicate a point of equivalence, that is, a value of the added moles of titrant that is stoichiometrically equivalent to the moles of the substance of unknown concentration, are required (LibreTexts). The pH of the solution must change during the acid-base titration; in particular, it increases if NaOH of unknown concentration is added to a standard acid. The change in pH is a metric that helps determine if the equivalence point has been reached with an indicator. Phenolphthalein is used as the acid-base titration indicator because this indicator is extremely sensitive to an alkaline environment.
Procedure
This laboratory work was based on an experimental approach to quantitative analysis. In the first part of the experiment, KHP was used to standardize (determine the exact concentration) of NaOH. The precise amount of KHP (0.4-0.6 g) was dissolved in 25 mL of distilled water. Then, 25 mL of sodium hydroxide solution was transferred to a 400 mL beaker, stirred vigorously, and transferred to a vertical burette.
Titration procedures were performed in the three trials, and the specific amount of NaOH neutralizing phthalate to the point of equivalence was recorded in all three cases and then averaged. The equivariance point, that is, the stoichiometric equality of the moles of the two substances, was determined using phenolphthalein, which was added to the phthalate. When the equivalence point was reached, the indicator changed color. In the second part of the experiment, a standardized NaOH solution with a specific concentration was used to identify the concentration of commercial vinegar. This was done by titrating the standardized NaOH solution for vinegar of unknown concentration to determine the percentage error compared to the reported value on the commercial bottle.
Results
Table 1 shows the results of the primary NaOH standardization measurements using KHP. Table 2 shows the results of a second titration in which NaOH was used as the standard solution with which the vinegar concentration was determined. It shows the titration results for acetic acid, showing the averaged values. Table 3 shows the summarized results and calculations.
Table 1. Results of the primary measurement of NaOH titration
Table 2. Results of the primary measurements of vinegar titration
Table 3. Summary of data and results of calculations
Equations [1]- [7] show calculations for the first sample for demonstration purposes. Similar calculations were performed for each of the three samples.
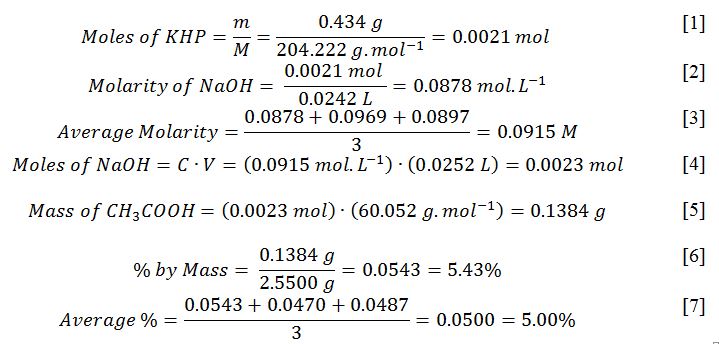
Conclusion
This work was concerned with determining the concentration of commercially available vinegar using two sequential titration procedures. In the first part, the concentration of NaOH was determined, and the average value was 0.0915 M. In the second part, NaOH was used to standardize the acetic acid, with an average concentration of 0.0500 or 5.00%. This corresponds to the stated vinegar concentration on the bottle, so the empirical data is as stated.
Works Cited
IU. “Buffers, Indicators, and Solution Standardization.” Indiana University, Web.
LibreTexts. “Acid/Base Titrations.” Libre Texts Chemistry, Web.