In the present experiment, we were randomly given one of three metals, which included two-valent cations of copper, cobalt, and nickel. These metals are capable of forming coordination centers, that is, combining with ligands to form complex compounds. Both inorganic and organic materials were used as ligands, including for example, deionized water, ammonia solution, EDTA, and pyridine. These salts differ in color, so the mixing process produced seven test tubes with complex salts of different colors, as shown in Figure 1. As can be seen, the color schemes of all the solutions were assigned to the blue spectrum, ranging from pale blue to deep purple.

The arrangement of the tubes in this order was compared with the corresponding arrangement in the peers in order to check the literacy of the color ranking and to identify the ligands without errors qualitatively. Comparison of the rainbow arrangement of the resulting solutions allowed the ligands to be identified in their association with the metal. In particular, copper cations were given as the metal. Table 1 demonstrates the ranking of the ligands in rainbow order (from red to purple) according to what coloration they gave in the compound with copper. In addition, a formula for each ligand is proposed to determine the presence of unshared electron pair donors for copper.
Table 1: Ranked List Of Ligands With An Indication Of Molecular Formulas Depending On The Color In The Complex Salt In Connection With Copper
It is noteworthy that the presence of nitrogen atoms in the ligand automatically intensified the blueness in the color of the corresponding complex compound; we can assume that increasing the amount of nitrogen was associated with the intensification of such color. Another assumption is the observation of the location of the nitrogen within the ligand. For the last three or four ligands, the nitrogen is located at the edge of the molecule, which probably facilitates binding to the copper coordination center due to the simplified formation of the donor-acceptor bond. In addition, there were no oxygen atoms in the most saturated solutions in which the ligands were pyridine, ammonia, and ethylenediamine — hence, it is likely to assume that oxygen reduces the intensity of the blue color in the complex compound.
For the first two test tubes, whose colors were pale blue and blue, a spectrometric analysis was performed. The difference in color between the two test tubes was due to the interaction of the copper coordination center with the ligands of water and potassium oxalate. We can assume that water is a weaker ligand than potassium oxalate, so the color intensity is different. Another reason is that both ligands are capable of forming stable complexes with copper; however, the structural nature of such salts is quite different. When exposed to electromagnetic radiation, the molecules are excited differently, which leads to the observed difference in color. In particular, UV radiation has been used to excite the absorption of excited molecules (Clark, 2022). In this sense, the transition to an excited state produces quantized energy, which can be easily determined if the corresponding wavelength for the compound is known. A table has been created for the ligands from the first two assays, allowing for a more meaningful examination of the differences.
Table 2: Comparison Of The First And Second Tubes
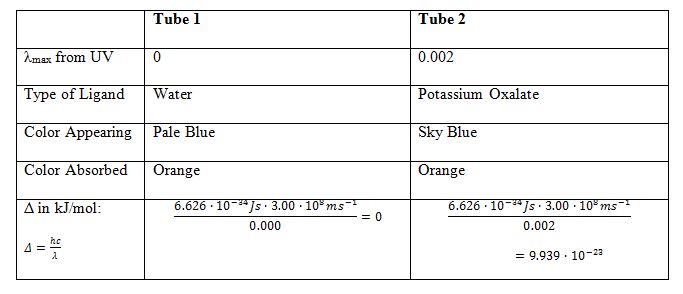
Consequently, the first tube contains a ligand with a stronger field.
Reference
Clark, J. (2022). What causes molecules to absorb UV and visible light. Chemistry
LibreTexts.