Introduction
When it comes to identifying a pathogen, whole-genome sequencing (WGS) can provide much useful information. This tool sequences the whole genome and compares it to the stored data of other sequenced genomes to find the specific pathogen among the identified matches. One of the most useful applications of the given approach is to determine the original source of an outbreak, and that is why WGS is actively used in epidemiology studies. Joensen et al. (2018) use WGS to analyse outbreaks of Campylobacter jejuni in the modern world by studying pathogen isolates. The scientists “support clonal linkage between isolates and highlight that outbreaks potentially occur more frequently than previously assumed” (Joensen et al., 2018, para. 14). It means that researchers should draw more attention to preventing and managing epidemics.
Simultaneously, Yang et al. (2017) use WGS to analyse the outbreak of Mycobacterium tuberculosis in Shanghai, China. The researchers stipulate that WGS “can be used to identify putative source cases, super-spreaders and transmission directions in the absence of, or complementary to, extensive epidemiological data” (Yang et al., 2017, p. 275). Even though these two examples demonstrate that WGS addresses human beings, the tool can also be used to investigate poultry pathogens.
Concerning chickens, spotty liver disease (SLD) is a severe problem. According to Van et al. (2017, p. 226), this condition leads to egg production losses and mortality in birds. That is why numerous studies have been conducted to identify the bacteria that is responsible for the given disease. Van et al. (2017, p. 226) mention that Campylobacter hepaticus is the cause of SLD in chicken, which is an initial hypothesis of the practical. However, additional research is needed to obtain more data on the SLD bacteria and their pathogenesis. Consequently, the aim of the paper is to isolate the unknown pathogen’s DNA from chicken with an SLD and use WGS to identify this specific pathogen. Thus, the Rapid Annotation using Subsystem Technology (RAST) will annotate bacterial genomes, and the Basic Local Alignment Search Tool (BLAST) will identify similarities between sequences.
Material and Methods
This work deals with an unknown bacterial sample that was obtained from chickens with SLD. As for methods, the given practical relies on Practical manual: whole-genome sequencing for identification and gene function prediction of bacterial genomes (2020). This document highlights all the steps required to decode a pathogen, including three separate practicals. Thus, Practical 1 explains tagmentation of genomic DNA, Practical 2 comments on library qualification and Practical 3 discusses bioinformatics issues. Consumables and equipment are mentioned at pages 6, 8 and 13 of the Practical manual (2020). Thus, it is possible to conclude that the given practical follows specific manual regulations.
Results
As has been mentioned above, the sequenced genome is submitted to the RAST for annotation and to the BLAST for identification. The given section is going to present the results of these procedures. Figure 1, a BLAST screenshot, demonstrates that the search has concluded that Campylobacter hapaticus strain HV10 is an SLD pathogen, while Campylobacter jejuni is the closest related species. The average nucleotide identity (ANI) value of the result is more than 99%.
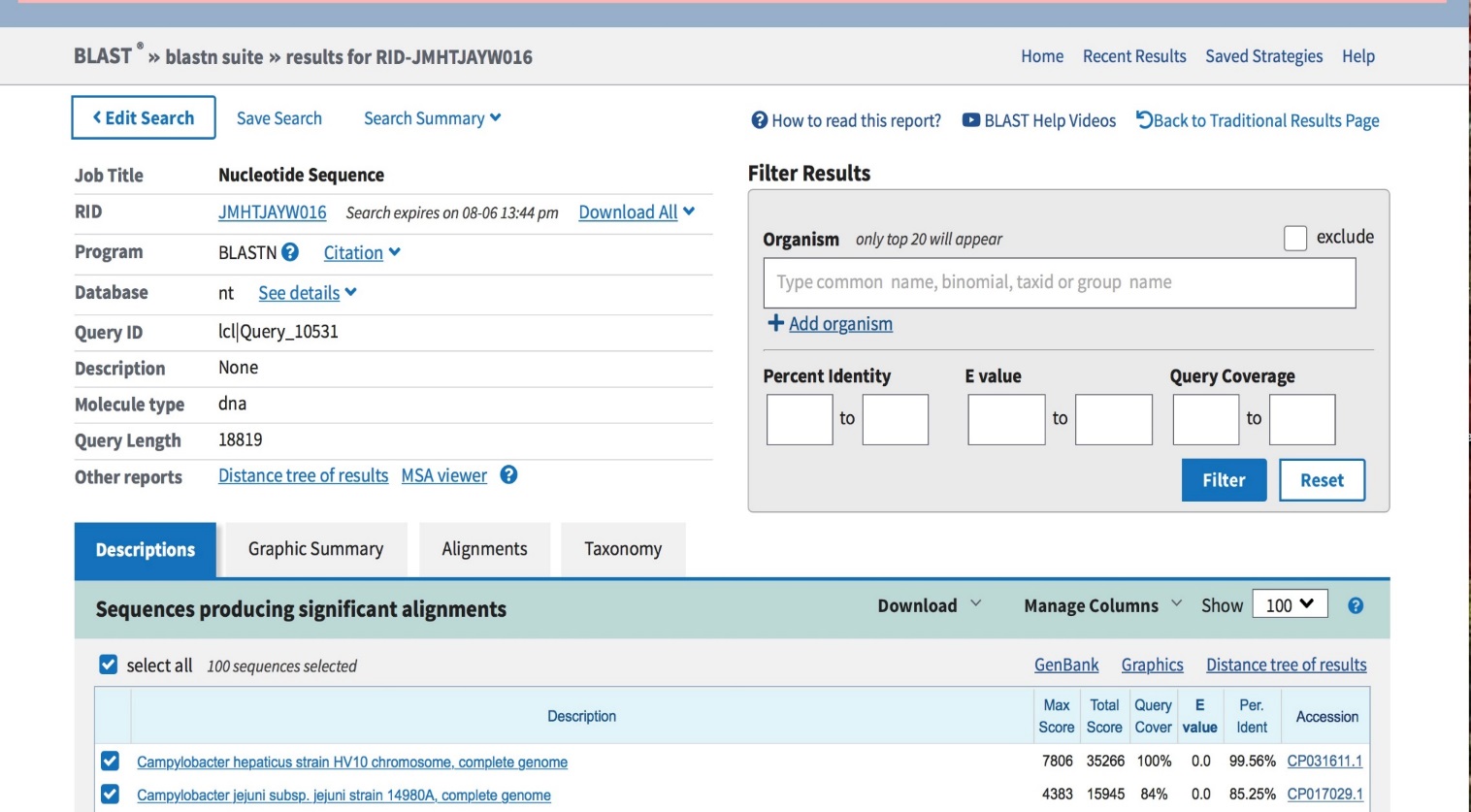
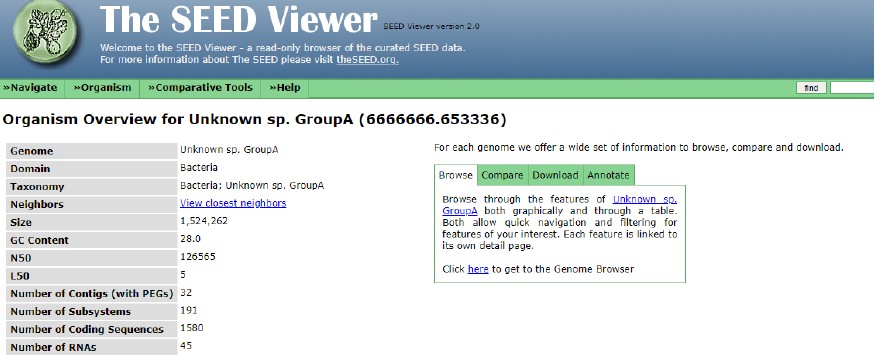
Figure 2 represents the Seed Viewer data for the annotated genome. This information is useful to define the number of coding sequences and RNAs of the given isolate. Thus, Figure 2 shows that the isolate has 1,580 coding sequences and 45 RNAs. It means that the sample under analysis implies many genes and RNAs that can be matched against the given database. This fact should increase the reliability of the obtained results.
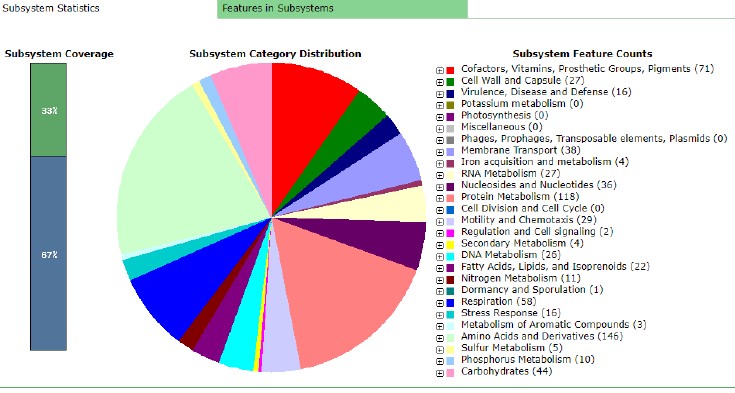
At the same time, Figure 2 indicates that the isolate has 191 subsystems, and Figure 3 below presents their analysis. The latter shows that the genome under investigation does not have any phages, prophages, transposable elements or plasmids. However, Figure 3 indicates 16 virulence, disease and defence genes. This subsystem is essential because it can include antibiotic resistance elements that, in turn, determine disease treatment options.
Discussion
WGS has demonstrated that Campylobacter hepaticus is an unknown pathogen that is isolated from chickens with an SLD. It is the same species as the bacteria that usually cause the disease. Thus, the practical results have supported the initial hypothesis and proved that Campylobacter hepaticus is a typical cause of an SLD among chickens. This finding is useful because it can help prevent SLD outbreaks and treat ill birds more successfully.
Since the identified isolate is one of Campylobacter species, it is necessary to comment on the genomics and proteomics features of the group. Thus, Van et al. (2019, p. 6) indicate that a typical structure of a Campylobacter genome includes “many genes encoding chemotaxis (11 genes), motility (47 genes) and adherence/surface protein (59 genes).” In addition to that, this genome acquires carbohydrates and metals with the help of appropriate metabolism loci (Van et al., 2019, p. 6). These are the features that are typical for any of the Campylobacter species.
However, Campylobacter hepaticus is a separate genome that implies unique coding sequences. Firstly, the given isolate has “genes with predicted roles in chemotaxis, capsule and lipooligosaccharide synthesis and metabolism” (Van et al., 2019, p. 6). It results in the fact that the pathogen tends to move from the gastrointestinal tract to the liver. Secondly, the lipooligosaccharide locus (LOS), a region that experience rearrangements, is unique to Campylobacter hepaticus because of “2 kb of the inserted sequence” (Van et al., 2019, p. 7). It means that the genome has a significant part that is unique compared to other Campylobacter species. Thirdly, it is reasonable to comment on antibiotic resistance of the isolate because this phenomenon determines treatment options. In general, the risk of antibiotic-resistant plasmids is present in bacteria isolated from poultry sources. However, the subsystem statistics above has indicated that the isolate does not have any plasmids but contains 16 virulence, disease and defence genes. These elements can be a source of antibiotic resistance, meaning that they deserve additional attention.
Conclusion
Whole-genome sequencing is a useful tool to analyse genomes and identify their specific pathogens. This approach is used in epidemiology because it helps to determine a virus or bacterium that has caused an outbreak. That is why this method is utilised to investigate chickens with spotty liver disease. The initial hypothesis stipulated that Campylobacter hepaticus causes this disease, but additional research was necessary to prove it. Thus, whole-genome sequencing has demonstrated that Campylobacter hepaticus is responsible for spotty liver disease outbreaks among poultry. Furthermore, the practical has shown that Campylobacter hepaticus has genes that lead to niche adaptation, colonisation and virulence. This information means that further research is needed to minimise the risk of Campylobacter hepaticus outbreaks.
References
Joensen, K. G. et al. (2018) ‘Whole-genome sequencing of Campbylobacter jejuni isolated from Danish routine stool samples reveals surprising degree of clustering’, Clinical Microbiology and Infection, 24(2), pp. 201.e5-201.e8.
Practical manual: whole genome sequencing for identification and gene function prediction of bacterial genomes (2020).
Van, T. T. H. et al. (2017) ‘Campylobacter hepaticus, the cause of spotty liver disease in chickens, is present throughout the small intestine and caeca of infected birds’, Veterinary Microbiology, 207, pp. 226-230.
Van, T. T. H. et al. (2019) ‘Survival mechanisms of Campylobacter hepaticus identified by genomic analysis and comparative transcriptomic analysis of in vivo and in vitro derived bacteria’, Frontiers in Microbiology, 10, pp. 1-19.
Yang, C. et al. (2017) ‘Transmission of multidrug-resistant Mycobacterium tuberculosis in Shanghai, China: a retrospective observational study using whole-genome sequencing and epidemiological investigation’, The Lancet Infectious Diseases, 17(3), pp. 275-284.