Thalassemia
Thalassemia entails a series of inherited disorders caused by one or more mutations.
The mutations occur on the globin genes of haemoglobin, leading to a lack of or decreased synthesis of equivalent globin chains.
There are over 400 specific alterations on the globin chains, causing various groups of disorders, and the effects of the mutation depend on the globin chain affected (Randolph).
A decrease in the number of global chains available reduces haemoglobin assembly, and consequently, the red blood cells.
The patients may develop mild or severe forms of the defects, and the former is asymptomatic, while the latter expresses several symptoms.
Classification of Thalassemia
The classification of thalassemia is based on the type of globin chain affected.
Usually, the important thalassemia revolved around the α- and β-chains.
This is because the α- and β-chains account for over 97% of the normal haemoglobin in adults (Randolph).
On the one hand, α-thalassemia is caused by the absence or decreased production of the α-globin chains.
On the other hand, β-thalassemia is due to the lack of or decreased production of the β-globin
Demographics
Thalassemia is considered a group of genetic defects that affect people worldwide.
In every year, between 100,000 to 200,000 children are born with severe forms of thalassemia, and around 60,000 of them have β-thalassemia (Randolph)
According to Randolph, α –thalassemia occurs in 20% and 6-11% of Southeast Asia immigrants and African Americans in North America, respectively.
However, most of these people are carriers, and they do not exhibit the symptoms of thalassemia.
In Thailand, there are over 3,000 births of people who have mutations on both chains (Randolph).
α –thalassemia
α –thalassemia disorders result from a mutation, which occurs on chromosome number 16, and each of the diploid chromosomes has two α –genes (Randolph).
The mutations cause a reduction in the production of the α –chains, and the alteration can affect one or more genes, leading to four genetic disorders.
A condition called hydrops fetalis develops when all four genes are deleted. It develops when both patients have α –thalassemia and the child born either die or are born with complications, which are detrimental to their health and that of the mothers (Zhan et al. 1).
If only three α –genes are deleted, haemoglobin H disease develops.
The disorder α –thalassemia minor develops when only two genes are deleted, while the deletion of one gene leads to a condition referred to as a silent carrier.
β-thalassemia
Unlike α –thalassemia, β-thalassemia involves two β-globin located, one on each chromosome.
Additionally, most of the mutations are non deletional, causing several clinical severities.
Most of these defects are due to point mutations on the DNA region controlling the expression of β-genes.
The conditions are classified either based on the genotype or phenotype.
The genotype classification system depends on the mutation’s impact on the production of β-globin, and there are two groups: β+ and β0 (Origa 609).
In phenotypic classification, there are four groups, and they include β-thalassemia minor, β-thalassemia major, β-thalassemia minima, and β-thalassemia intermedia.
Pathophysiology
Usually, the maturing red blood cells in the body synthesize the α- and β-chains on the ratio of 1:1 (Randolph).
However, if this ratio is not adhered to, leading to a lack or decreased synthesis of the chains, the production of the other chain will be in excess.
The imbalance interferes with haemoglobin production from erythrocytes, causes chronic haemolysis, and erythropoiesis becomes defective.
In cases where the α-chains are produced in excess, insoluble precipitates are formed within the cells.
The precipitates then adhere to the cell membrane, damaging it, and consequently, apoptosis follows.
Macrophages then destroy the erythroblasts filled with precipitates, leading to defective erythropoiesis.
The spleen also removes the affected cells from circulation leading to extravascular haemolysis.
Clinical Picture
One of the major clinical findings in thalassemia is anaemia. It is due to increased extravascular haemolysis and defective erythropoiesis. The image above demonstrates microcytic, hypochromic anaemia in patients with haemoglobin H disease (Randolph).
Its severity among patients vary, and it depends on the mutation and the number of genes involved.
Enlargement of the spleen is also common due to its role in extravascular haemolysis. However, in some cases, the load becomes too heavy on the spleen, and it becomes compromised, leading to functional hyposplenism.
Besides, chronic haemolysis causes the liver to excrete excess bilirubin, which leads to the formation of gallstones.
Clinical Finding
There is increased erythropoiesis in the bone marrow to replace the destroys erythrocytes, hence, erythroid hyperplasia.
In extreme cases, the bone marrow expands, and there is remodelling of the bone, leading to skeletal defects and pathological fractures.
Moreover, the increased erythropoietic activity causes an increase in demand for iron.
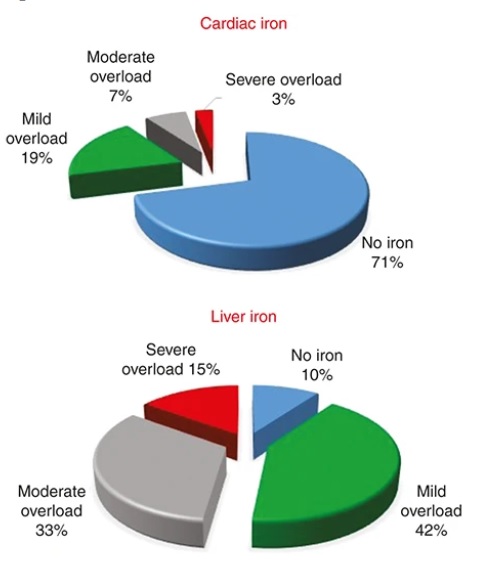
This results in elevated levels of iron absorption, and it occurs in excess. There is ineffective incorporation of the surplus iron, leading to its accumulation on macrophages located in the spleen, liver, and the bone marrow, and eventually, to the parenchymal cells of various organs. The image shows iron levels in the heart and liver, depending on the overload.
With time, the iron levels become toxic and affect various organs, including the pituitary gland and the liver.
Several defects develop, and examples are arrhythmias, cardiomyopathies, hypogonadism, cirrhosis, and growth failures (Randolph).
Laboratory Techniques and Findings
Usually, the peripheral blood results help in guiding the diagnosis of the disease. The haematocrit and haemoglobin levels are reduced, and so are the MCH, MCV, and MCHC levels.
For instance, in β-thalassemia, haemoglobin levels can drop to 20-30g/dL (Randolph).
The Mentzer technique is used to differentiate between iron deficiency anaemia and thalassemia since both conditions cause microcytic hypochromic anaemia. Results of a value more than 13 indicate iron deficiency, while a value of less than 13 favour thalassemia.
Another technique used is haemoglobin electrophoresis. In this method, HbF and HbA2 are decreased in cases of α –thalassemia and increased in β-thalassemia.
Screening programs such as PCR-based nucleic acid assays are used in areas where thalassemia are common.
Management
Severe cases of hemoglobin H disease can be managed by performing splenectomy or long-term transfusion therapies. Splenectomy is done in cases of increased possibilities of pulmonary hypertension, venous thrombosis, and infections (Olga 615).
These transfusions prevent the development of effects of chronic severe anemia such as stunted growth.
Iron chelation therapy is also indicated in cases of iron toxicity, and it includes drugs such as deferaserox or deferipone (Randolph). There are also oral chelators, but they are associated with agranulocytosis, arthropathy, and neutropenia.
Folate supplementation is also necessary, as the bone marrow is overworked due to increased erythropoiesis.
When there is no response to these treatment options, a bone marrow transplant is indicated in severe cases.
Generally, therapy should start as early as possible to avoid the manifestation of thalassemia.
Works Cited
Origa, Raffaella. “β-Thalassemia. “Genetics in Medicine”, vol. 19, no. 6, 2017, pp. 609-619.
Randolph, TIM R. “Thalassemia. Clinical Laboratory Hematology. Edited by Shirlyn B, McKenzie and Lynne Williams, Pearson, 2015.
Zhan, Wenli, et al. “Comparison of Cord Blood Hematological Parameters Among Normal, α-thalassemia, and β-thalassemia Fetuses Between 17 and 38 Weeks of Gestation.” Scientific Reports, vol. 11, no. 1, 2021, pp. 1-10.