Introduction
The latent heat of fusion, or enthalpy of fusion, determines the amount of energy that must be transferred to a solid to cause it to undergo physical changes associated with aggregate state transitions. The transition temperature from solid to liquid state for each substance is unique and is determined by the molecular and physical properties of the sample itself (Madhu, 2021). This laboratory work examines the example of ice that is immersed in water in a calorimeter chamber, a process that results in an abrupt change in temperature and the formation of thermal equilibrium.
Data
In this experiment, information was collected regarding the mass of the calorimeter and bowl (total), the mass of the empty calorimeter, the water, and the contents: all raw data are shown in Table 1.
Table 1. Raw data obtained during the experiment.
In addition, the temperature dynamics of the ice-water mixture in the calorimeter bowl during each thirty seconds was also studied: Table 2 contains information about this.
Table 2. Data on temperature dynamics over time.
Results
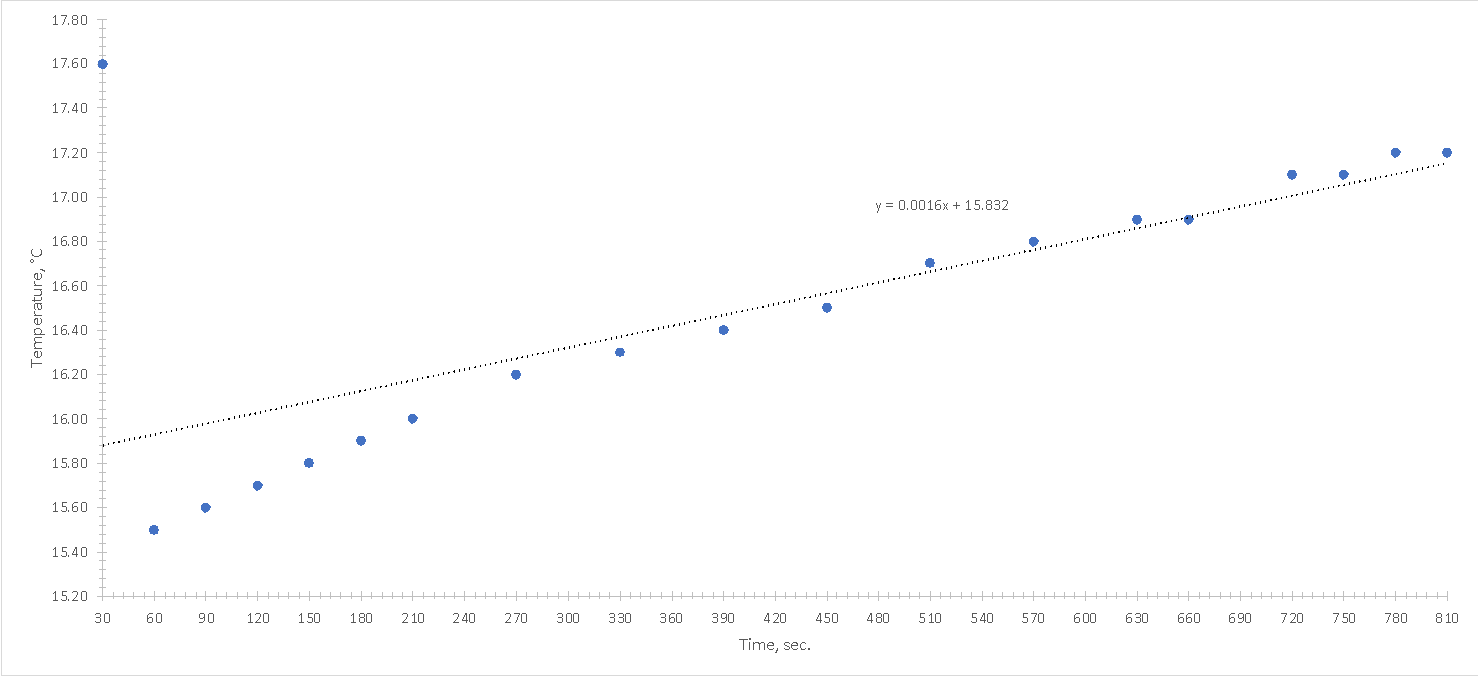
Based on the results from Table 2, a visualization of the temperature versus time was plotted. As shown in Figure 1, the temperature of the mixture slowly increased over time, although initially there was an extremely sharp drop during the first thirty seconds from 17.6 °C to 15.5 °C. The graph shows that the initial temperature for the process was 17.6 °C and the final temperature, when the graph could be said to have plateaued, was about 17.4 °C. This means that the equilibrium temperature was almost equal to the initial temperature, but it also decreased by 1.14 percent.
In addition, the latent heat of melting (Lf) for ice can be calculated from the data in Table 1. To do this, the following formula is used:
Or:
This expression can be extended to:
Now it is possible to substitute the known values:
Then the percentage error:
Analysis
The sharp drop (Fig. 1) is due to the rapid transfer of temperature from water to ice as part of the heat transfer in an attempt to establish thermal equilibrium. Theoretically, the graph is expected to plateau further, which means that the temperature will not change. Such a condition is observed when equilibrium is reached, when the rates of both directions of heat transfer turn out to be identical. When water was added to the calorimeter, but no ice was added yet, the mass of the calorimeter cup and water was 0.1912 kg. When ice was added, the mass increased by 0.2212, indicating that 0.0300 kg of ice was added to the cup. The masses of the ring, lid and large cup were not taken into account, because these masses are constant throughout the experiment and no effect on the final masses, so these constant values could be excluded.
It is noteworthy that in the calorimeter setup there was no heat exchange between the mixture of water and ice and the room, according to the assumption. That is, all of the heat generated by this process affected only the thermometer, and no heat escaped to the outside environment. In the absence of this assumption, one would have to count the heat loss, but this was deliberately omitted. Accordingly, the sharp temperature loss during the first thirty seconds corresponded to the transfer of heat only from water to ice, and no heat escaped outside the calorimeter chamber. In this case, as follows from the graph, thermal equilibrium is reached at a certain point, which means that the rates of heat energy transfer of the two oppositely directed processes are identical.
Conclusion
In this paper, the latent heat of melting for ice was investigated using a calorimeter setup. The results showed that the temperature of water dropped sharply when ice was placed in it, after which it slowly plateaued, indicating the possibility of thermal equilibrium between ice and water. At the same time, the calculated value of the latent heat of melting differed from the reference value by 37.5%, which indicates a moderate error. This error could have been caused by calculation errors or inaccuracies in starting the experiment. Despite this, the experiment can be considered successful because it demonstrated the possibility of calculating the enthalpy of melting for solids.
Reference
Madhu. (2021). What is the difference between latent heat of fusion and solidification? DB.