Abstract
This study dedicated to the estimation of the benthic ecosystem biodiversity and its dependence on environmental features of the ecosystem. To estimate the diversity, macro-invertebrates’ orders were identified in samples. It is known that the composition of benthic invertebrates strongly depends on the characteristics of the environment. This approach is widely used to evaluate the current state of different freshwater ecosystems. In this investigation, it was shown that the diversity of benthic macro-invertebrates was different for different riffle and run sites. Evenness and richness of the invertebrates’ orders were calculated. It was shown that richness of the diversity was from low to moderate, and evenness was moderate for the analyzed riffle. It could be concluded that this riffle was under moderate stress conditions.
Introduction
Benthic macro-invertebrates are organisms that live in freshwater (rivers, lakes, streams) sediments. The macroinvertebrate community consists of diverse orders of multicellular animals (Benthic Invertebrate Communities, 2017). The structure of the community strongly depends on features of the environment, in particular, sediments and water quality, hydrological and physical characteristics: temperature, transparency, salinity, and mineral composition, and others (Queirós et al., 2015).
Freshwater biodiversity is affected by numerous factors, including human-related activities. Nowadays, a lot of freshwater ecosystems are exposed to anthropogenic stress due to water pollution, eutrophication, soil erosion, and fine sedimentation deposition (Leitner et al., 2015). Changes in environmental characteristics lead to changes of macro-invertebrates diversity of a particular ecosystem. Therefore, diversity measurement is a useful tool for the determination of the ecosystem state (Stoll et al., 2015).
Evenness and richness of species are important characteristics of the ecosystem. Both features depend on environmental conditions. It was claimed that stress factors might lead to the richness decline. Some species might disappear because of non-appropriate conditions (Murphy and Romanuk, 2014). Besides, evenness in the stressful environment might also be rejected. The reason for it is the following: some species might be more adaptive than others. Therefore, some of them might be overrepresented in the ecosystem, while others might be underrepresented (Lemieux and Cusson, 2014).
The research hypothesis is the following: environmental stress leads to the increase of the proportional presence of tolerant orders and a decrease of general biodiversity (Passy et al., 2017).
To check the research hypothesis, the following aim of the study was determined: to estimate the dependence of benthic macro-invertebrates diversity of the ecosystem and estimate their evenness and richness.
Materials and Methods
Results
In the study, the diversity of orders of benthic macro-invertebrates was determined in five river riffle sites and four-run sites. To determine the difference between total benthic the macro-invertebrates orders’ distribution and the distribution in the analyzed riffle, the comparison of different orders’ percentage was performed. The results are presented in Table 1.
Table 1. Percentage of different orders of the total numbers of organisms for each riffle and totally.
Totally, eighteen macro-invertebrate orders were identified in analyzed riffle and run benthos sites. In the riffle #4, seven orders of macro-invertebrates were presented: Coleoptera, Plecoptera, Megaloptera, Trichoptera, Ephemoptera, Diptera, and Nematoda. It could be stated that orders were presented not equally. It was also estimated that, in general, the diversity of benthic macro-invertebrate was higher in riffles (totally, 18 orders) than in runs (totally, 11 orders).
The percentage ratio of different orders presented in riffle #4 is shown in Figure 1. According to it, the majority of organisms belonged to three orders: Trichoptera (41.5%), Ephemoptera (34.1%), and Coleoptera (17,4%), followed by Diptera (4.7%), Nematoda (1.7%), and Plecoptera and Megaloptera (0.3% both).
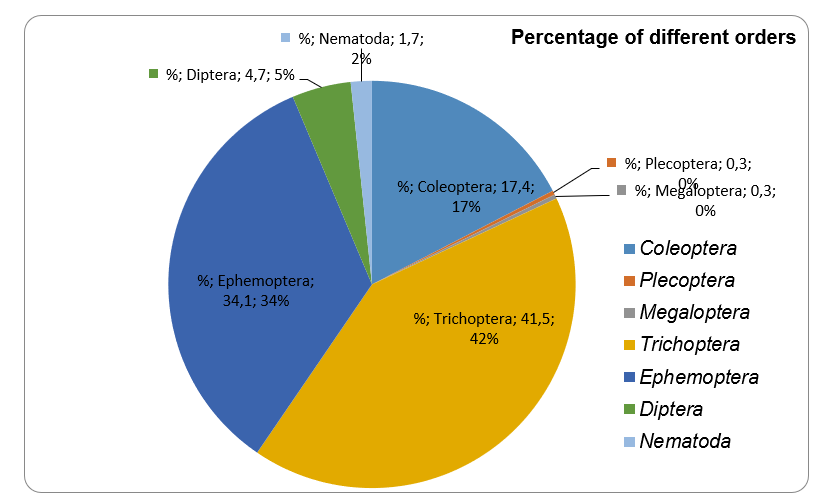
It was shown in Table 1 that different orders presented not equally in different riffles. The percentage of different invertebrates’ orders in riffle #4 is different than the total distribution. In particular, Coleoptera organisms covered 10.30% of total samples but 17.39% of the riffle #4 total samples. Besides, the percentage of Coleoptera species is higher in the riffle #4, in comparison with all other riffles. Similar to it, Trichoptera species covered a higher percentage among the riffle #4 samples (41.47%), in comparison to the total percentage (32.5%) and other riffles percentage (from 19.86 to 37.4%).
On the other hand, some orders were not identified or presented in fewer amounts in the riffle #4 benthic samples, even though they are presented in other samples. In particular, Odonata species covered 2.11% of total samples but were not found in riffle #4. Diptera samples covered 4.68% of the riffle #4 samples, in comparison to 6.74% of the total numbers of invertebrate organisms.
Identically, the percentage of different orders’ in runs # 1-4 and in total was calculated. The results are presented in Table 2.
Table 2. Percentage of different orders of the total numbers of organisms for each run and totally.
According to Table 2, totally, different orders presented in samples not equally. More than a half of all organisms (53.99%) were identified as Ephemoptera, followed by Diptera (12.21%), Trichoptera (10.33%), Coleoptera (7.98%), Mollusca (7.51%), and Odonata (3.76%). Each other order covered less than 1% of the total samples. Similar to the riffles results, different orders of benthic macro-invertebrates presented in different runs not equally. Some of them are presented in all analyzed run sites (Coleoptera, Ephemoptera, Diptera). In contradiction to it, Decapoda samples were presented just in the run #2, Amphipoda samples were found just in run #4, and Plecoptera samples were identified in the run #3. In general, it is difficult to estimate the regularity of the orders’ distribution.
Discussion
In the study, it was estimated that different macroinvertebrate orders were distributed among the different analyzed riffles and runs’ sites in a non-equal manner. It means that the environmental conditions of measured sites were different. In general, it was estimated that orders’ diversity is higher in riffle sites in comparison to run sites which are corresponded to the literature data (Niba and Mafereka, 2015). It could be explained by the higher concentration of nutrients in the riffle area.
To evaluate the state of the ecosystem, it is important to estimate its richness and evenness. A Shannon-Weiner Index is commonly used for the ecosystem’s richness measurement (Alve et al. 2), and a Simpson Index is an approach to calculate the evenness of taxonomic groups (Belley and Snelgrove, 2017). To estimate the evenness and richness, a Simpson’s Index and a Shannon-Weiner Index were calculated. The results of the calculation are shown in Table 3.
Table 3. Simpsons Index (D) and Shannon-Weiner Index (H) calculation
Simpson Index calculation was the following:
- Simpson Index D is the sum of square values of relative abundances. D = (0.34)2+ (0.41)2+ (0.04)2+ (0.02)2+ (0.17)2+ (0.003)2+ (0.05)2+ (0.003)2= 0.32
- Simpsons Diversity Index= 0.32
- Simpsons Inverse Diversity Index= 1-0.32 or 0.68
- Simpsons Reciprocal Diversity Index = 1/0.32 = 3.13
- ED= 1/d/s Where 1/D= 3.13 and S is 7, therefore, ED= 3.13/7 = 0.45
The interpretation of the results is the following: D=0.32 means from low to moderate diversity. According to the literature data, low diversity is more typical for the environment under the stress conditions. Therefore, it could be supposed that riffle #4 is characterized by moderate environmental stress which reduces the level of diversity.
A Simpson Index can also be used to estimate the evenness. A score of ED = 0.45 is more close to the middle (maximum value is 1) which represents moderate evenness. In the riffle #4, three orders were more represented, and four were less represented. More represented orders were Trichoptera, Ephemoptera, and Coleoptera
Shannon-Weiner Index was also calculated to estimate evenness. Calculation formulas were presented in Table 3. It was estimated that evenness (H) was 1.286, while the maximum evenness (HMax) was 3.618. Therefore, the results of the Shannon-Weiner Index calculation correspond with the results of the Simpson Index estimation. Evenness of macro-invertebrates orders in the riffle #4 was moderate. Therefore, it could be stated that not all orders are represented equally in the ecosystem: three of them are overrepresented in comparison to the other four. It could be supposed that these three orders are more adaptive and/or tolerant to the environmental conditions of the riffle #4. Thus, it could be concluded that the research hypothesis was confirmed by the obtained data.
Works Cited
Alve, E., S. Korsun, J.Schönfeld, N. Dijkstra, E. Golikova, S. Hess, and G. Panieri. 2016.Foram-AMBI: a sensitivity index based on benthic foraminiferal faunas from north-east atlantic and arctic fjords, continental shelves and slopes. Marine Micropaleontology 122: 1-12. Web.
Belley, R. and P.V.R. Snelgrove. 2017. The role of infaunal functional and species diversity in short-term response of contrasting benthic communities to an experimental food pulse. Journal of Experimental Marine Biology and Ecology 491: 38-50. Web.
Benthic Invertebrate Communities 2017. RAMP. Web.
Leitner, P., C. Hauer, T. Ofenböck, F. Pletterbauer, A. Schmidt-Kloiber, and W. Graf. 2015. Fine sediment deposition affects biodiversity and density of benthic macroinvertebrates: a case study in the freshwater pearl mussel river waldaist (upper Austria).” Limnologica-Ecology and Management of Inland Waters 50: 54-57. Web.
Lemieux, J. and M. Cusson. 2014. Effects of habitat-forming species richness, evenness, identity, and abundance on benthic intertidal community establishment and productivity.” PloS One 9(10): 261-271. Web.
Murphy, G.E.P., and T.N. Romanuk. 2014. A meta‐analysis of declines in local species richness from human disturbances. Ecology and Evolution 4(1): 91-103. Web.
Niba, A.S. and P.S. Mafereka. 2015. Benthic macroinvertebrate assemblage composition and distribution pattern in the upper Mthatha river, Eastern Cape, South Africa. African Journal of Aquatic Science 40(2): 133-142. Web.
Stoll, S., P. Breyer, J.D. Tonkin, D. Früh, and P. Haase. 2016. Scale-dependent effects of river habitat quality on benthic invertebrate communities—implications for stream restoration practice. Science of the Total Environment 553: 495-503. Web.
Passy, Sophia I., Marius Bottin, Janne Soininen, and Helmut Hillebrand. 2017. Environmental filtering and taxonomic relatedness underlie the species richness–evenness relationship. Hydrobiologia 787(1): 243-253. Web.
Queirós, A.M., N. Stephens, R. Cook, C. Ravaglioli, J. Nunes, S. Dashfield, C. Harris, G.H. Tilstone, J. Fishwick, U.Braeckman, and P.J Somerfield. 2015. Can benthic community structure be used to predict the process of bioturbation in real ecosystems? Progress in Oceanography 137: 559-569. Web.