Introduction
A calorimetric experiment under isolated conditions is conducted to determine the amount of heat given off by an object according to thermodynamic concepts. The second law of thermodynamics states that heat is transferred from a hotter body to a cooler body, which is used as the foundation for the calorimetric analysis (Lotha, 2023). Tests were performed for two metal objects, which included placing them alternately in the calorimeter and evaluating the maximum temperature reached. The purpose of the work was to calculate the amount of heat required to change the temperature of each metal object, using mathematical formulas to describe the calorimetric process. The results demonstrated that the specific heat capacity of the second metal cylinder was approximately 3.5 times higher than that of the first.
Data
Table 1 shows the transcribed primary data collected in this lab work and used for analysis.
Table 1. Results
Results
Calculation
Under the conditions of an isolated calorimeter setup, the following equality is true:
∑ Q = 0
The total heat is made up of the heat of the calorimeter, the metal object (cylinder), and water, then:
Qs+Qc+Qw = 0
Or in more detail:
(c•m•ΔT)s+(c•m•ΔT)c+(c•m•ΔT)w=0
In this equation, all variables except the specific heat capacity of the cylinder, cs, are known. Specifically, the specific heat capacity of water was taken as 4182 J.kg-1.°C-1, and the specific heat capacity of the calorimeter made of aluminum was 900 J.kg-1.°C-1. The masses and temperature differences were determined from the data shown in Table 1. Therefore, for the first metal cylinder, the specific heat capacity can be calculated as shown in [a]:
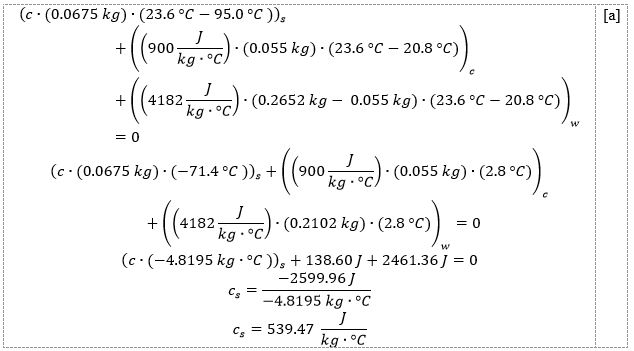
And for the second metal cylinder, the calculation is shown in [b]:
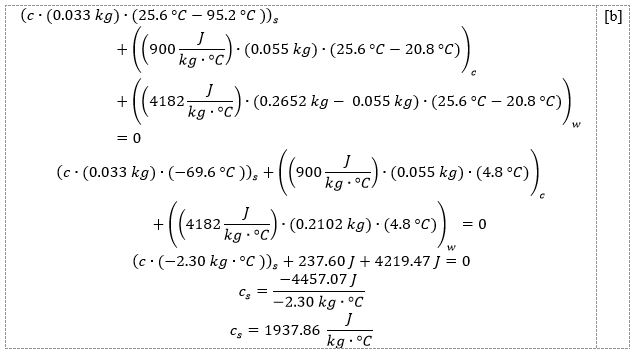
Thus, the specific heat capacity of the first metal cylinder was 539.47 J.kg-1.°C-1, and the second was 1937.86 J.kg-1.°C-1, almost 3.5 times higher. The results are affected by the uncertainty of measurement because uncertainties are inherent in the way the mass and temperature are measured. The higher this uncertainty, the lower the accuracy of the results.
Conclusion
In the laboratory work, two calorimetric analyses were performed to determine the specific heat capacity of two metal cylinders. This procedure can be considered a method of indirect qualitative analysis because, by knowing the reference values of the specific heat capacity, it becomes possible to determine the nature of the material. The results indicated the heat absorption rate of the first metal object was 539.47 J.kg-1.°C-1, and the second was 1937.86 J.kg-1.°C-1.
Consequently, a comparison of these values with the reference ones can be useful in determining the material of each of the cylinders. In general, the laboratory work can be considered successfully completed since both the practical part of the experiment and the theoretical part of the calculations have been fulfilled, and the set goal has been achieved.
Reference
Lotha, G. (2023). Second law of thermodynamics. Britannica. Web.