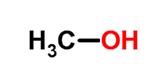Objective
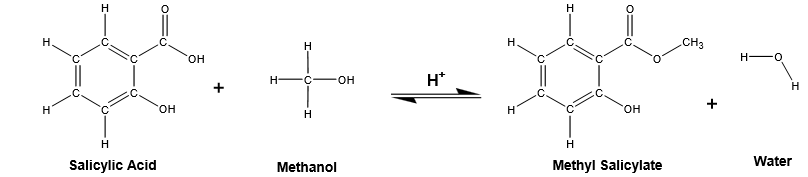
The objective of this experiment was to synthesize methyl salicylate (Oil of Wintergreen) from salicylic acid and methanol using sulfuric acid as a catalyst. The ester synthesis was achieved through the reflux Fischer esterification process. The compound was identified using IR and NMR spectra.
Introduction
Esters are aromatic compounds used in various fields such as the cosmetic industries due to their fragrance. Alcohol combines with a carboxylic acid to form an ester in a process referred to as Fischer esterification. For the reaction to yield at a preferable rate, an acid catalyst must be used. One of the major drawbacks in esterification is its acid-catalyzed process that happens at equilibrium. Therefore, to drive the reaction to favor product formation, one reactant must be in excess.
Or
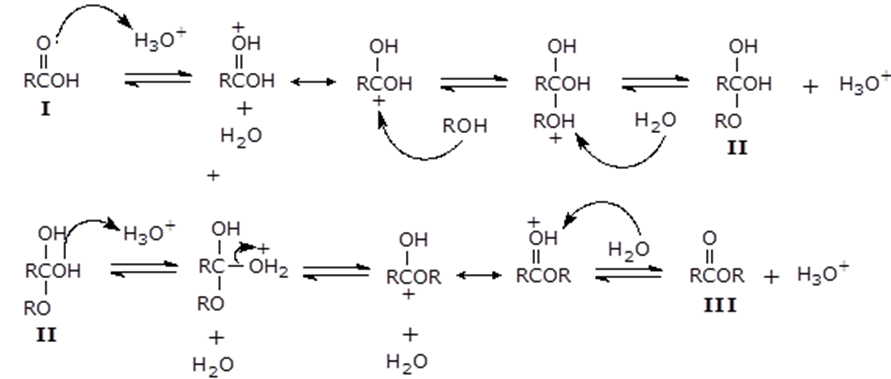
Reactants and Products
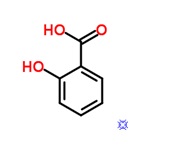
Salicylic Acid
Methanol
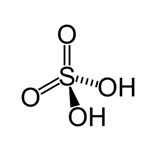
Sulfuric Acid
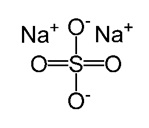
Sodium Sulfate
Dichloromethane
Sodium Bicarbonate
Methyl Salicylate
Procedure
First, a 50 mL round bottom flask was weighed to determine its actual weight. 4.9 g of salicylic acid was put into the flask followed by the addition of 12.5 mL methanol. The mixture was swirled to dissolve the acid after which the weight of the flask and its contents was taken. 5.0 mL of concentrated sulfuric acid was added to the mixture in small portions with careful swirling. The mixture was heated on a reflux condenser with continuous swirling for 45 minutes.
An oil layer that formed was separated after the product was allowed to cool to room temperature. 10 mL of ice water was added, and the contents transferred to a separating funnel. The oil was extracted using 15 mL dichloromethane and washed with 15 mL water and 15 mL of 5% aqueous sodium bicarbonate solution.
The organic layer obtained was dried over sodium sulfate and decanted into a pre-weighed 100 mL round-bottom flask. The solvent was removed by careful vaporization in a rotary evaporator. The weight of the final product was weighed to determine the percentage yield. Ultimately, the product was analyzed using IR and NMR.
Calculation of Theoretical and Percentage Yield
Theoretical yield
Mass of Methanol = 12.5 ml x 0.7918 g/ml = 9.89g
Moles of Methanol = 9.89g /32.04 g/mol =0.30867 mol of Methanol
Moles of salicylic acid = 4.9 g /138.12 g/mol =0.03547 mol of salicylic acid.
Therefore, salicylic acid is the limiting reactant
0.03547 mol of salicylic acid = 0.03547 mol of methyl salicylate
Percent Yield
Theoretical yield = (0.03547 mol *152.15 g/mol) = 5.4 g
Empty round flask = 62.5 g
Round flask+ product = 66 g
Mass of the product = 66g – 62.5g = 3.5g
Percent yield = (Actual yield/ theoretical yield) * 100
(3.5 g/5.4g) *100= 64.815 %
Results
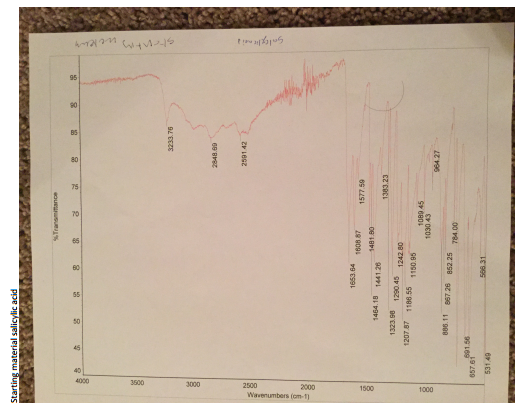
The methyl salicylate formed was the oil part of the product. The weight of the product was 3.5 g compared to a theoretical yield of 5.5g. Therefore, the percentage yield was 64.815%. The IR spectrum of the starting material had two distinct functional groups, which were the phenol and carboxylic acid. Three main stretching bands were seen. The stretch at 1653.64 cm-1 indicated the carbonyl group while the 3233–3000 cm-1 stretch indicated the O-H phenol. One other stretch at 886 cm-1 also indicated O-H phenol.
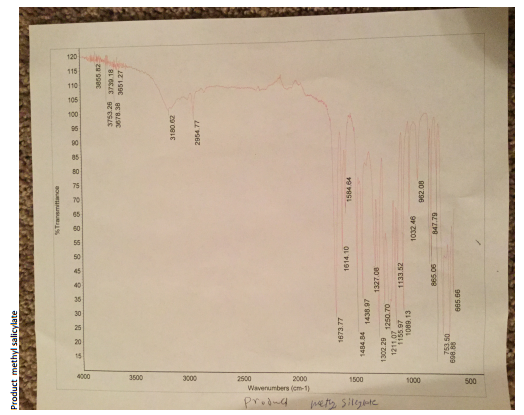
The IR spectrum of the product showed a new stretching band at 1673 cm-1 indicating the carbonyl group (C=O) of the ester. The O-H carboxylic acid (3000–2500 cm-1, 886 cm-1) as well as the C=O carboxylic acid (1652 cm-1) stretching bands that were present previously in the reactant were absent in the spectrum of the product.
On the NMR spectrum, the singlet peak between 3 and 4 ppm indicated the presence of 3 hydrogen ions (protons) attached to carbonyl group (C=O)-O-CH3). The doublet peaks at 7 ppm indicated the two protons of the aromatic ring. A singlet peak around 10-11 PPM indicated 1 proton of (-OH) group.

Discussion
The synthesis of methyl salicylate was an esterification process involving the reaction of salicylic acid with methanol to form methyl salicylate and the concomitant elimination of water.
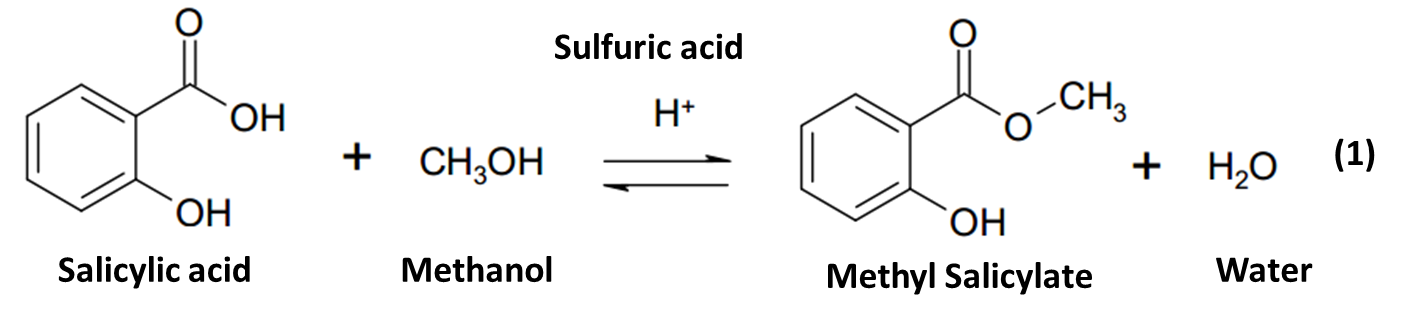
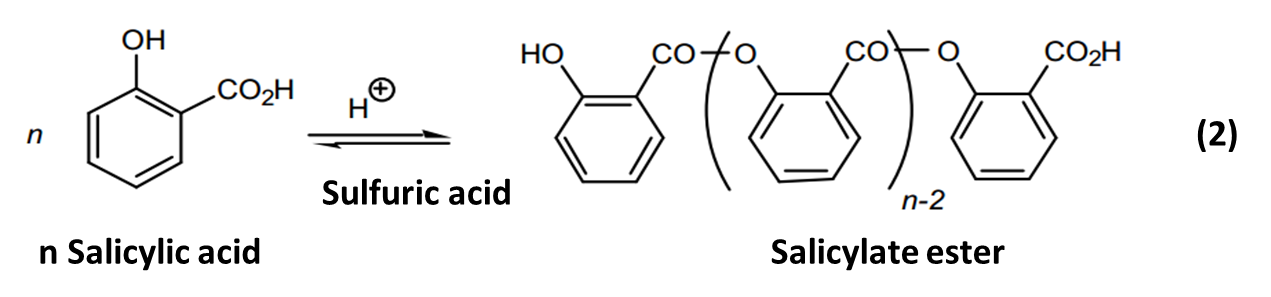
Or
The mechanism of action of sulfuric acid is as shown below.
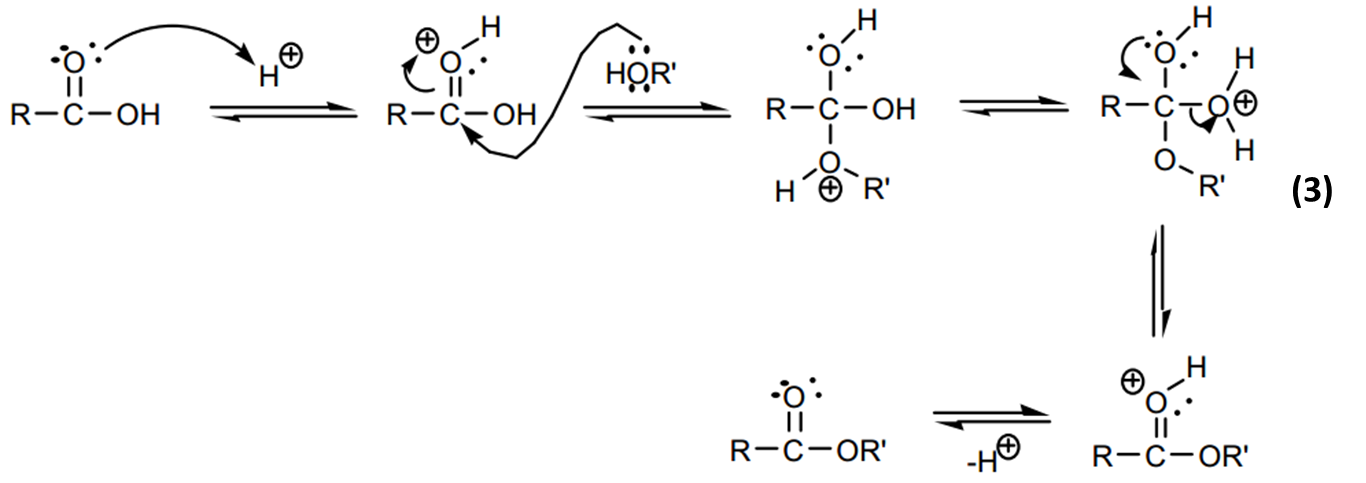
Concentrated sulfuric acid (H2SO4) was used as a catalyst. The acid promoted the nucleophilic substitution of acyl group on the carboxylic acid by protonation of the carbonyl carbon, which then became an electrophile that reacts with the alcohol. Sulfuric acid was also used to lower the transition energy for the intermediate and activation energy needed for elimination of water (Burrows et al., 97).
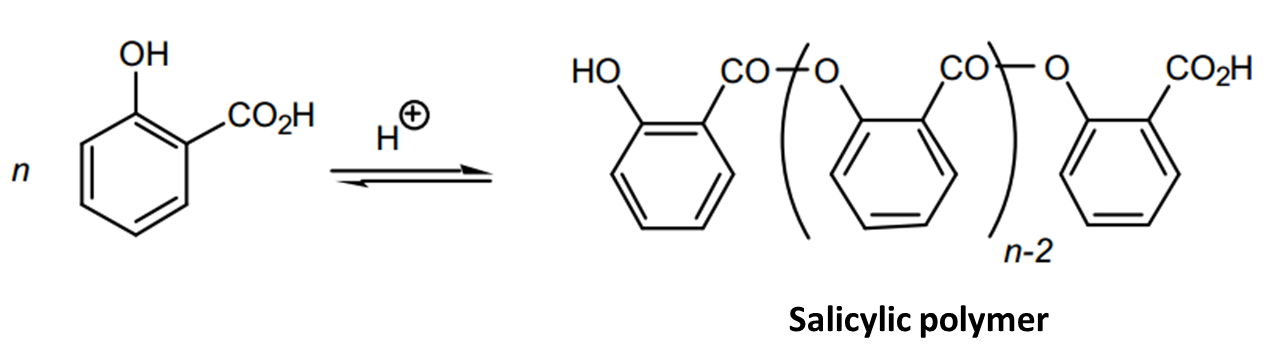
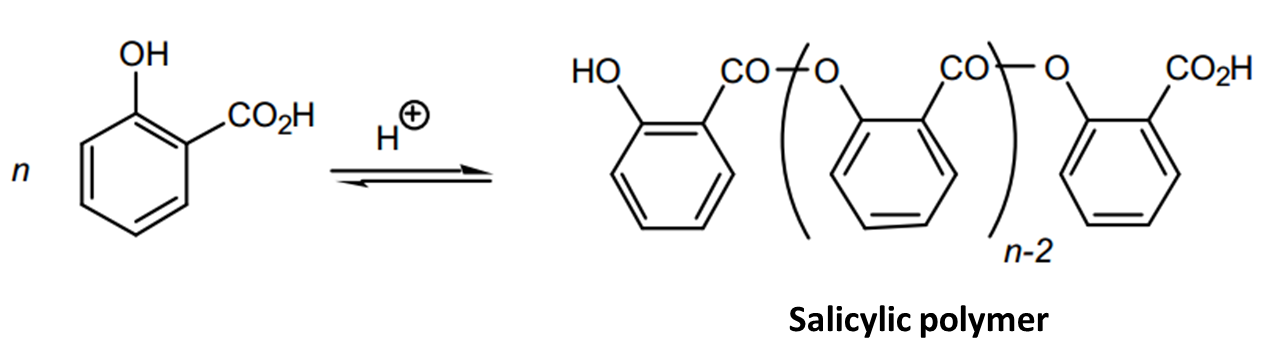
Salicylic acid had a carboxylic acid group and the alcohol group, and esterification could occur at either site. When methanol was used, esterification occurred at carboxylic group to form methyl salicylate ester. Salicylic acid reacted with more salicylic acid on the phenol group to form salicylate ester. The salicylate esters formed a white precipitate that was separated from methyl salicylate by the addition of dichloromethane.
The experiment involved heating the solvent at its boiling point for a prolonged period during the reaction process. While this process could be achieved in a flask or a beaker exposed to open air, there were numerous hitches with such methods. For example, the molecules of the organic solvent (methanol) could diffuse into the air before the reaction ends thus reducing the concentration of the reactants. Methanol is very flammable, and the accumulation of methanol vapor in the atmosphere could lead to an explosion or fire.
Additionally, methanol vapor could cause methanol poisoning. Therefore, heating in a closed environment was necessary, which was achieved using heating reflux where heating was achieved in a closed environment (Claridge 25). The organic solution alongside two to three boiling stones (also called boiling chips) was placed in a round-bottom flask and clamped to a ring stand. The boiling chips being porous, inert materials created a steady stream of small air bubbles when heated. The bubbles streams ensured the solution boiled evenly and prevented the solvent from becoming superheated.
A condenser, which contained a large water jacket with running cold water, was attached to the flask to promote the condensation of the solvent vapor as it rose from the flask during the heating process. When reflux heating was complete, the cooled liquid from the flask was decanted into a clean container.
There was a need to crystallize the product, which was achieved through the addition of ice water. Ice water crystallized the methyl salicylate formed leaving behind the methanol solvent. Extraction is the process of moving a solute from one solution to another solution in which it is more soluble. The extraction of methyl salicylate was achieved by dissolving the solution into a methylene chloride (dichloromethane) solution and separating the layers using a separating funnel to separate two immiscible liquids.
In this case, dichloromethane was used to remove methyl salicylate from the unreacted excess methanol. Running two small extractions was better than one big extraction to ensure that the two layers were completely separated without losing any of the product.
Methyl salicylate dissolved in dichloromethane to form a separate organic layer. The stopcock was opened, and the aqueous layer was allowed to flow first and collected in a different container. The process was repeated severally time for the aqueous layer to ensure there was no organic layer (methyl salicylate) was left. When dichloromethane was added to the organic solution, it separated into organic layer and aqueous layers which had different densities. The bottom layer, which had a higher density of 1.174 g/mL, was the organic layer comprising methyl salicylate. Conversely, the top layer with a lower density of 0.791 g/mL contained water, sulfuric acid, and un-reacted methanol.
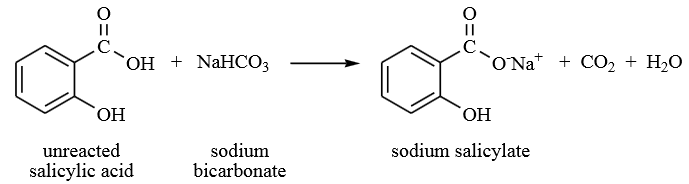
During the esterification process, not all the salicylic acid reacted with methanol. Therefore, water was first added to remove the acid followed by the addition of sodium bicarbonate to remove any residual acid. Sodium bicarbonate reacted with the unreacted salicylic acid to form sodium salicylate, water and carbon dioxide gas as by-products. The production of carbon dioxide gas was observed as fizzing during the process. The sodium salicylate produced formed the aqueous layer and was removed from the mixture leaving the methyl salicylate in the organic layer.
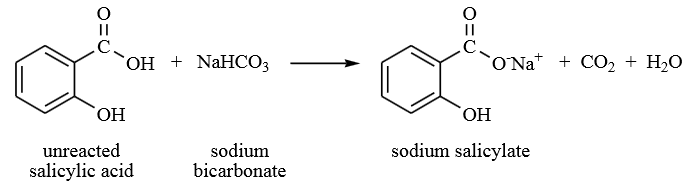
It was necessary to eliminate water from the organic solution formed. The most common way of removing water is by use of inorganic drying agents. Anhydrous sodium sulfate was used as the drying agent, which became hydrated when it came into contact with water in the organic solution. The hydration of the salt led to the removal of water from the solution.

After the elimination of water, the “wet” solution appeared cloudy while the “dry” solution appeared crystal-clear. To separate the two, gravity filtration was done using fluted paper. Gravity filtration is a method used to remove solid impurities from an organic solution. These impurities include drying agents or undesirable side product or leftover reactants. The fluted filter paper was folded into a cone shape and fitted on a stemmed glass funnel, which was fitted into a flask. The solution was passed through the fluted paper leading to the collection of the organic solution in the flask.
Finally, the solvent was evaporated using a rotary evaporator. Rotary evaporation is an advanced method of evaporation under reduced pressure. In a rotary evaporation, the solvent-solute mixture was placed in a round-bottom flask attached to the rotary evaporator. The flask was lowered into a warm water bath, and the motor was engaged, causing the flask to rotate. A vacuum was then applied to the system. The reduced pressure lowered the boiling point of the solvent, whereas the rotation increased the surface area thus allowing more molecules of the solvent to enter the vapor state.
The solvent vapors that evaporated were condensed back to the liquid state in the condenser and collected in a separate flask. To ensure that there was no loss of product via evaporation, a small content of the solvent was left in the flask. The power was turned off, and the remaining solvent was allowed evaporating to near dryness. The apparatus was disassembled to enable the collection of the dry methyl salicylate from the flask by scraping with a spatula.
To analyses the structures of the reactant and the product, IR and NMR techniques were used. IR elucidates the structure of a compound by analyzing the absorbance of electromagnetic radiations (Hesse, Meier and Zeeh 115). The IR spectrum of the starting material had two distinct functional groups namely a carboxylic acid and a phenol.
The IR spectrum of the starting material had two distinct functional groups, which were the phenol and carboxylic acid groups. Three main stretching bands were seen. The stretch at 1653.64 cm-1 indicated the carbonyl group while the 3233–3000 cm-1 stretch indicated the O-H phenol. One other stretch at 886 cm-1 also indicated O-H phenol. The IR spectrum of the product showed a new stretching band at 1673 cm-1 indicating the carbonyl group (C=O) of the ester.
The O-H carboxylic acid (3000–2500 cm-1, 886 cm-1) as well as the C=O carboxylic acid (1652 cm-1) stretching bands that were present previously in the reactant were absent in the spectrum of the product. This observation could be attributed to the formation of the ester, which contained the carbonyl functional group only. Other peaks on the IR results were due to the presence of the solvent and other impurities such as the unreacted methanol and salicylate acid.
The product was also analyzed using nuclear magnetic resonance (NMR), which was a technique that utilized electromagnetic energy to identify a compound (Claridge 35). The difference in energy between the two states creates gave a resonance line, which was characteristic of a particular proton. These resonance lines are plotted relative to a standard reference compound tetramethylsilane [(CH3)4Si, TMS], which was the prototype at zero point (Mokhatab and Poe 48). On the NMR spectrum, the singlet peak between 3 and 4 ppm indicated the presence of 3 hydrogen ions (protons) attached to carbonyl group (C=O)-O-CH3). The doublet peaks at 7 ppm indicated the two protons of the aromatic ring. A singlet peak around 10-11 PPM indicated 1 proton of (-OH) group. Therefore, the NMR analysis confirmed that methyl salicylate, an ester, was part of the products (Pretsch, Buhlmann, and Badertscher 85).
The experiment yielded 3.5g as opposed to the anticipated theoretical yield of 5.4g, which translated to a percentage yield of 64.815%. The low percentage yield was attributed to several factors the first one being the polymerization of the acid. During the synthesis of methyl salicylate, other side reactions could take place. Salicylic acid had a carboxylic acid group and a phenol group. Therefore, esterification could occur at either site.
When salicylic acid reacted with methanol, not all the acid formed an ester with the alcohol. Some of the acids polymerized at the carboxylic acid group to form a polymer, seen as a white solid. The second reason was that not all reactants were used up in the reaction leading to the low yield. During the extraction process, some of the product was lost. Also, when methylene chloride was dried over anhydrous sodium sulfate and then decanted, that not all the product was transferred (Cann and Verhulst 275).
Nevertheless, the achievement of 64.815% of the product could be considered a success and indicative of the effectiveness of the experimental procedure. Several problems were usually encountered in esterification reactions. For example, obtaining a 100% yield was unattainable due to the equilibrium of the reaction. To circumvent this problem, several interventions were considered such as the removal of water from the reaction as it was formed. However, water had a higher boiling point than ester and alcohol and could also form azeotropes with the alcohol and carboxylic acid, which could complicate its distillation.
To overcome this problem, excess methanol was used to achieve maximum yield. Extraction of the product was another major problem that occurred with esterification. The conventional experimental way of using Pasteur pipet could lead to the loss of a high amount of the product. To curb this problem, gravity filtration method was used.
Conclusion
The synthesis of methyl salicylate in this experiment was successful as indicated by the IR and NMR analysis and a percentage yield of 64.815%. Despite the challenges encountered in the experimental process, it was concluded that esterification was a reliable method of synthesizing esters from alcohols and carboxylic acids.
Pre-Lab Questions for Exp. 8 – Esterification Reaction
Give the overall reaction for the synthesis of methyl salicylate from salicylic acid.
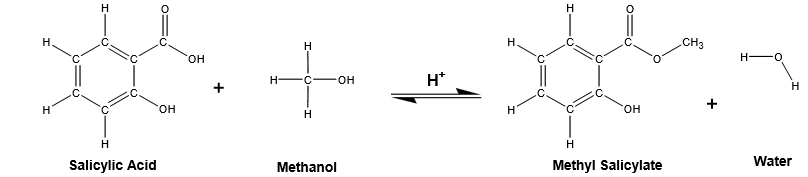
Using the amounts of the starting materials (Blackboard procedure), give the theoretical yield of the reaction. What is the limiting reactant?
- Mass of Methanol = 12.5 ml x 0.7918 g/ml = 9.89g
- Moles of Methanol = 9.89g /32.04 g/mol =0.30867 mol of Methanol
- Moles of salicylic acid= 4.9 g /138.12 g/mol =0.03547 mol of salicylic acid Therefore, 0.03547 mol of salicylic acid = 0.03547 mol of methyl salicylate
- Theoretical yield = (0.03547 mol *152.15 g/mol) = 5.4 g
Therefore, salicylic acid is the limiting reactant.
During the work-up of the reaction, aqueous sodium bicarbonate is used to wash the organic layer to remove impurities. What two compounds are removed from the organic layer during this process?
One of the impurities compound removed from the organic layer is unreacted salicylic acid.
The other impurity is the polymer of salicylic acid.
Oil of wintergreen (methyl salicylate) is a liquid. How can you determine when most of the methylene chloride has been evaporated via rotary evaporation?
Rotary evaporation is based on the differences in boiling point of the organic solutions involved. The boiling point of methylene chloride is 39.75oC compared to the boiling point of methylene salicylate, which is between 220 and 224 oC (is higher). This boiling point difference can be utilized to separate the two solvents. When there is no more vapor at the boiling point of methylene chloride the evaporation is considered complete.
Works Cited
Burrows, Andrew, John Holman, Andrew Parsons, Gwen Pilling, and Gareth Price. Chemistry³: Introducing Inorganic, Organic and Physical Chemistry, Oxford: Oxford University Press. Print.
Cann, Howard M. and Henry L. Verhulst. “The salicylate problem with special reference to methyl salicylate.” The Journal of Pediatrics 53.3 (1958): 271-276. Print.
ChemSpider 2016. Methanol.Web.
Claridge, Timothy DW. High-resolution NMR techniques in organic chemistry, New York: Elsevier, 2008. Print.
Hesse, Manfred, Herbert Meier and Bernd Zeeh. Spectroscopic Methods in Organic Chemistry, New York: Thieme, 2014. Print.
LearnChemistry 2016. Salicyclic Acid. Web.
Mokhatab, Saeid and William A. Poe. Handbook of Natural Gas Transmission and Processing, Waltham, MA: Gulf Professional Publishing, 2012. Print.
Pretsch, Ernö, Philippe Buhlmann, and Martin Badertscher. Structure Determination of Organic Compounds, Berlin: Springer, 2009. Print.
PubChem 2016. Dichloromethane. Web.
PubChem 2016. Methyl Salicylate. Web.
PubChem 2016. Sodium Bicarbonate. Web.
PubChem 2016. Sodium Sulfate. Web.
PubChem 2016. Sulfuric Acid. Web.