The Objective
The objective of the experiment is to demonstrate the synthesis of methyl salicylate, which is an ester, through the process of esterification. In the experiment, salicylic acid reacts with methanol in the presence of concentrated sulfuric acids to produce methyl salicylate and water as the only products.
Esterification Reaction
Physical Properties of Reactants and Products
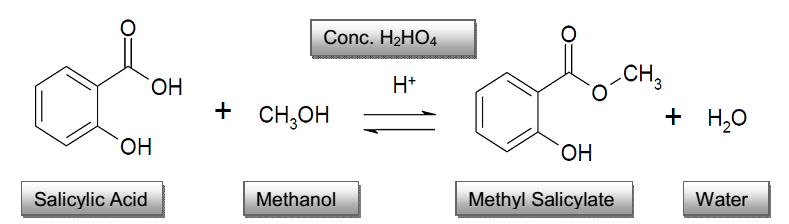
Salicylic Acid
Molecular formula: C7H6O3
Molecular weight: 138.12 g/mol
Molecular structure: Salicylic acid Melting point: 158-161°C
Boiling point: 211°C
Water solubility: 1.8 g/L
Density: 1.4 g/ml
Hazards: Harmful and highly flammable
(“Chemical Book: Product Chemical Properties” par. 1)
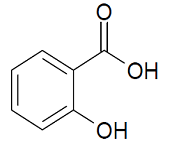
Methanol
Molecular formula: CH4O
Molecular weight: 32.04 g/mol
Melting point: -98°C
Molecular structure: Methanol
Boiling point: 65.4°C
Water solubility: Miscible
Density: 0.791 g/ml
Hazards: Harmful, highly flammable, and toxic
(“Chemical Book: Product Chemical Properties” par. 3)
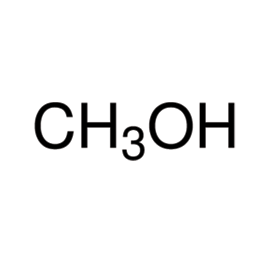
Sulfuric Acid
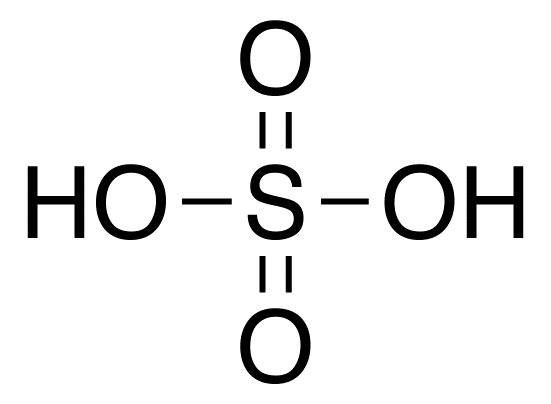
Molecular formula: H2SO4
Molecular weight: 98.08 g/mol
Molecular structure: Sulfuric acid
Melting point: 10°C
Boiling point: 290°C
Water solubility: Soluble
Density: 1.84 g/ml
Hazards: Harmful, highly flammable, toxic, and corrosive
(“Chemical Book: Product Chemical Properties” par. 4)
Methylene Chloride
Molecular formula: CH2Cl2
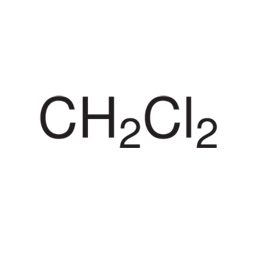
Molecular weight: 85.94 g/mol
Melting point: -97°C
Molecular structure: Methylene Chloride Boiling point: 39.8-40°C
Water solubility: Soluble
Density: 1.325 g/ml
Hazards: Harmful
(“Chemical Book: Product Chemical Properties” par. 1)
Sodium Bicarbonate
Molecular formula: NaHCO3
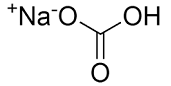
Molecular weight: 84.01 g/mol
Melting point: 300°C
Boiling point: 851°C
Molecular structure: Sodium bicarbonate Water solubility: 0.09/ml
Density: 2.16 g/ml
Hazards: Harmful and irritant
(“Chemical Book: Product Chemical Properties” par. 2)
Anhydrous Sodium Salicylate
Molecular formula: C7H5NaO3
Molecular weight: 160.103 g/mol
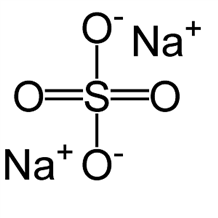
Melting point: 884°C
Boiling point: 1700°C
Water solubility: 0.09/ml
Density: 2.68 g/ml
Molecular structure: Sodium carbonate Hazards: Irritant
(“Chemical Book: Product Chemical Properties” par. 4)
Methyl Salicylate
Molecular formula: C8H8O3
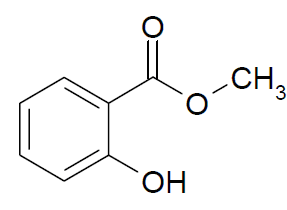
Molecular weight: 152.15 g/mol
Melting point: -5°C
Boiling point: 219°C
Molecular structure: Water solubility: Soluble
Density: 1.174 g/ml
Hazards: Harmful
(“Chemical Book: Product Chemical Properties” par. 3)
Water
Molecular formula: H2O
Molecular weight: 18.02 g/mol
Melting point: 0°C
Molecular structure: Water Boiling point: 100°C
Water solubility: Miscible
Density: 1 g/ml
Hazards: Corrosive
(“Chemical Book: Product Chemical Properties” par. 3)
Procedure
To commence the experiment, 4.9 grams of salicylic acid was weighed accurately and placed in a 50 ml round-bottom flask. Subsequently, 12.5 ml of methanol was measured, added to the 50 ml flask containing salicylic acid, and swirled until all the solids dissolved. 5 ml of sulfuric acid was measured and gradually added to the solution in the 50 ml flask while swirling and taking great caution. Once the addition of sulfuric acid was complete, a reflux condenser was attached to the flask and the mixture was heated under reflux for 45 minutes. As the layer of oil formed on top of the flask, the contents were swirled intermittently during the reflux period.
After the expiry of 45 minutes, the reaction mixture was then cooled to room temperature. Then, 10 ml of ice water was measured and added to the contents of the flask before transferring them to a separatory funnel. The product (oil of wintergreen) was extracted with two portions of 15 ml each of methylene chloride and was washed gently. The methylene chloride extracts were washed consecutively with 15 ml of water and subsequently by 15 ml of 5% aqueous sodium bicarbonate solution, which caused fizzing to occur. The organic layer formed was dried over sodium sulfate, decanted into a pre-weighed 100 ml round-bottom flask, and eventually evaporated using a rotary evaporator. Since the product formed is oil, caution was necessary to prevent the evaporation of the solvent together with the oil. The product formed was weighed and the percent yield determined. The analysis of the product was done by running the IR and NMR.
Results
Calculation of the theoretical and percent yield
Determination of the limiting reactant
Moles of salicylic acid: 4.9g/138.12 g/mol= 0.035 moles
Mass of methanol: 12.5 mL* 0.791 g/mL= 9.88 g
Moles of methanol: 9.88 g/ 32.04 g/mol= 0.308 moles
Therefore, salicylic acid is the limiting reactant
Theoretical yield
The reaction ratio of salicylic acid and methyl salicylate is 1:1
Actual yield
Mass of empty vial = 62.5 g
Mass of the vial + product = 66 g
Mass of the product = 66 g – 62.5g = 3.5 g
Percent yield
% Yield = Actual yield/theoretical yield × 100
% Yield= 3.5 g/5.3g × 100 = 66.03%
Synopsis of Results
The determination of the limiting reactant between salicylic acid and methanol formed the basis of calculating the theoretical yield. In this case, salicylic acid is the rate-limiting reactant because it has a lower number of moles than methanol. The reaction ratio of the reactants and products in the experiment shows that one mole of salicylic acid reacts with one mole of methanol to produce one mole of methyl salicylate and one mole of water. In this view, 0.035 moles of salicylic acid produce 0.035 moles of methyl salicylate, which give a theoretical a mass of 5.3 g. The actual yield of the product is 3.5 g, which represents 66.03% of the theoretical yield. The actual yield obtained was less than the theoretical yield due to some errors in the experimental process. One possible source of error is that all salicylic acid might not have reacted with methanol. Another possible source of error is that inefficient heating under reflux might have caused the product to evaporate. Moreover, washing of the product might have led to its loss through the aqueous phase.
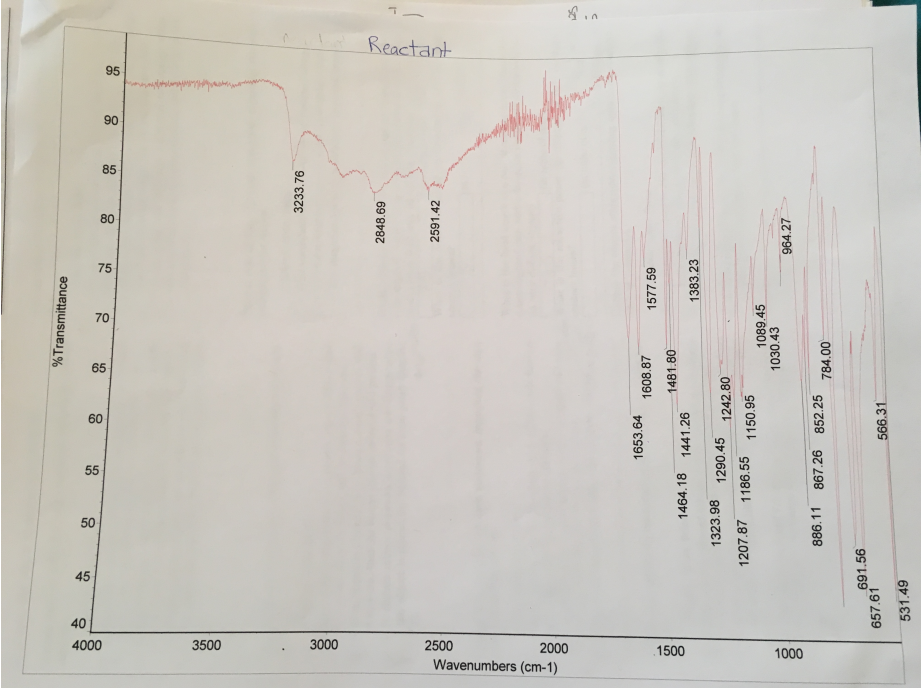
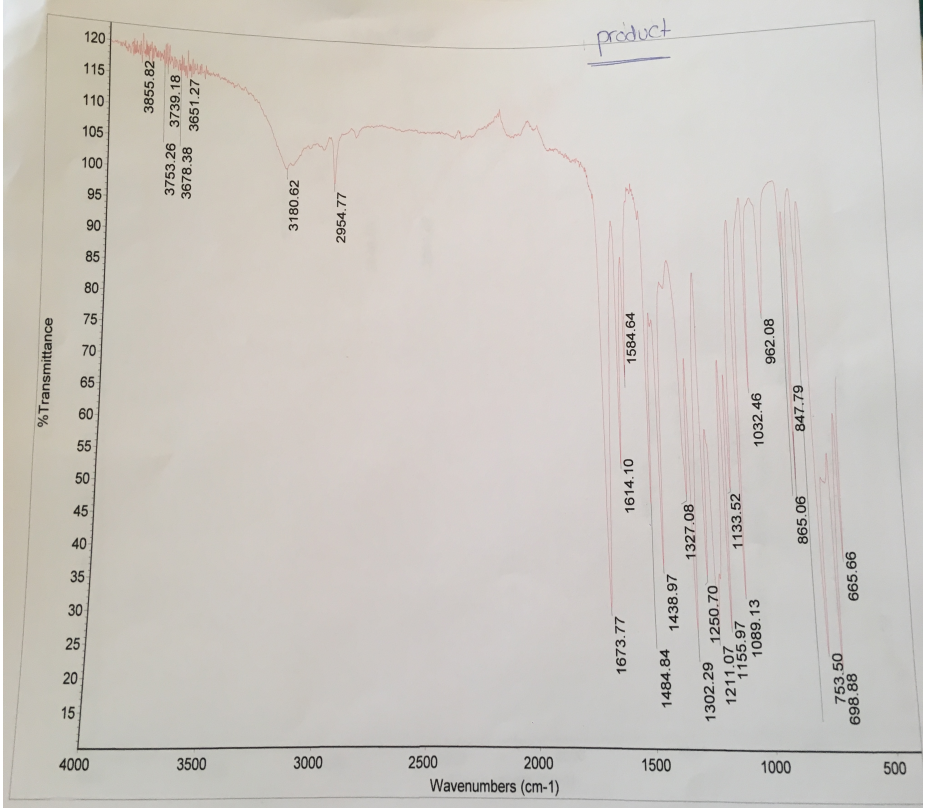
Comparative analysis of IR spectra of the reactants and the products show that they exhibit different peaks. The difference in peaks shows that the esterification reaction occurred. The reactant has a broad O-H stretch of carboxylic acid (salicylic acid) at 2848.69 cm-1 and 2591.42 cm-1, and O-H stretch of alcohol (methanol) at 3233.76 cm-1 (Figure 2). Comparatively, the product has lost the broad O-H stretch of carboxylic acid (salicylic acid) at 2591.42 cm-1 and retained the O-H stretch at 3180.62 cm-1 and 2954.77 cm-1. Moreover, C=O stretch of ester (methyl salicylate) emerged at 1673.77 cm-1. Hence, the IR spectra confirm the formation of methyl salicylate from the reaction of salicylic acid and methanol.
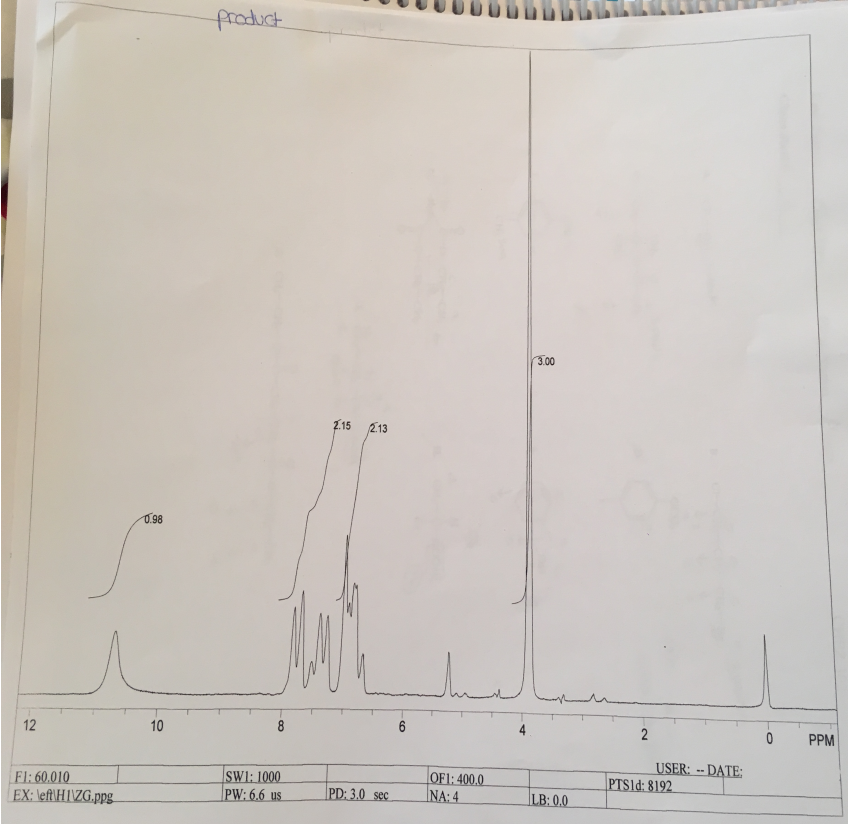
The NMR analysis shows that the spectra exhibit chemical shifts of methyl salicylate. The spectra have four peaks, which are 0.98, 2.15, 2.13, and 3.0. The peak of 0.98 at 10.4 PPM is singlet that represents a proton on the –OH group while the peaks between 6-8 PPM are two peaks, 2.15 and 2.13, which are doublet peaks that reflect two protons. At PPM 4, the spectrum has singlet peak (3.0), which represents three protons of the methyl group of the ester.
Discussion
The reaction of salicylic acid and methanol is a condensation reaction because there is the formation of methyl salicylate and water. The reaction of carboxylic acid and alcohol leads to the formation of esters in a process called esterification. The mechanism of the reaction is the replacement of the –OH (hydroxyl) group of the carboxylic acid with the –OR (alkoxy) group of the alcohol. In this case, the –OH group of salicylic acid was replaced with the –OR of methanol resulting in the formation of an ester called methyl salicylate. Concentrated sulfuric acid catalyzes the esterification process because it protonates the carbonyl group and creates primary carbocation, which resonates between the two –OH groups as shown in Figure 4. The lone pair of electrons in the –OH group of methanol initiates a nucleophilic attack on the carbocation. The formation of water molecule occurs when there is a proton transfer from the –OH group of methanol to the –OH group of salicylic acid, which create a good leaving group. Eventually, the deprotonation of the carbonyl group causes stabilization of the carbocation in the ester formed.
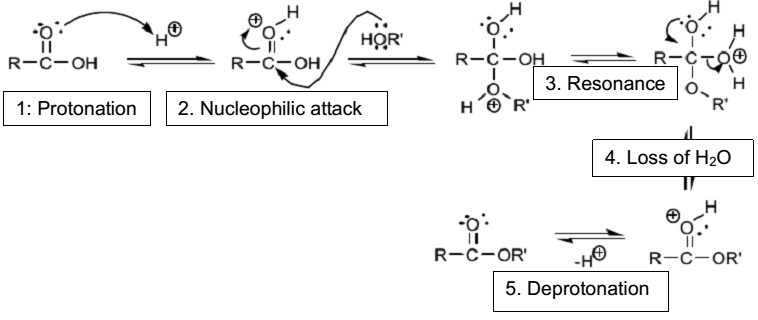
The concentrated sulfuric acid used in the experiment acts as a catalyst because it initiates the esterification reaction by speeding the reaction between salicylic acid and methanol. Fundamentally, sulfuric acid catalyzes the esterification reaction because it provides protons, which protonate the carbonyl group and creates a primary carbocation for –OH group of methanol to attack. The formation of carbocation is necessary for salicylic acid to react with methanol resulting in the formation of methyl salicylate and water as the only products. The addition of concentrated into the flask containing salicylic acid and methanol led to the formation of a white precipitate. As concentrated sulfuric acid cannot dehydrate methanol, it polymerizes salicylic acid resulting in the formation of a white precipitate. Hence, the white precipitate formed is a polymer of salicylic acid as shown in Figure 7.
Reflux is a technique in chemistry that allows heating of reacting contents to their boiling points in a closed system. The contents of the flask were heated at reflux for methanol and salicylic acid to react at optimal temperature. Moreover, heating under reflux happens in a closed system to prevent the loss of reacting contents since they evaporate and condense on cooler parts of the closed system. In essence, reflux is the distillation system that allows heating of reacting contents and condensation of vapor generated back to the reacting contents using the flow of cold water. Hence, the reflux system ensured that the reaction occurred optimally without the loss of some contents through the evaporation.
Once the refluxing mixture cooled, ice water was added and the contents transferred to the separatory funnel. The addition of ice water was necessary to stabilize the product formed, methyl salicylate, and prevent it from undergoing hydrolysis. Methyl salicylate can easily hydrolyze into methanol and salicylate if the conditions of the reacting contents do not favor forward reaction. Moreover, the addition of ice water prevented the aqueous and organic layers from mixing; hence, easing their separation in the separatory funnel.
Given that the contents in the separatory funnel comprise organic and inorganic phases, they form lower and upper layers based on their densities. The product, methyl salicylate, is in the lower layer because it is an organic compound with the density of 1.174 g/ml, which is denser than the aqueous layer with the density of 1.0 g/m. The aqueous layer has sulfuric acid, water, and the un-reacted traces of sulfuric acid and methanol.
During extraction, methyl salicylate was extracted with two portions of 15 ml methylene chloride. In extraction, double small extraction is better than a single big extraction because of the efficiency. The efficiency of the liquid-liquid extraction process is dependent on the equilibrium states between the solvents. In each equilibrium state, there is mass extraction. In this case, double small extraction creates two equilibrium states between the solvents leading to enhanced mass extraction. In a single big extraction, there is only one equilibrium state that allows mass extraction. Therefore, the number of equilibrium states created in an extraction process is commensurate to the efficiency of the extraction.
The methylene chloride extracts were combined and washed with water followed by sodium bicarbonate solution. Washing with water is important in removing excess water and other chemicals that are soluble in water (Pavia et al. 372). In this case, washing with water removed soluble species, which are methanol, salicylic acid, and sulfuric acid. Since washing with water leaves traces of acids (salicylic acid and sulfuric acid), washing with sodium bicarbonate neutralizes these acids. The gas that causes fizzing is the carbon dioxide produced by the neutralization reaction between acids and sodium bicarbonate. Fizzing stops when sodium bicarbonate solution has neutralized all the acids present in the flask. The following is the chemical equation for the neutralization equation:-
Acid + sodium bicarbonate → Salt + carbon dioxide gas + water
The net ionic equation: HCO3–(aq) + H+ (aq) → CO2 (g) + H2O (l)
The experiment used a drying agent in drying methyl salicylate. The use of drying agents is important because they selectively absorb water without affecting the state of other chemicals. Given that methyl salicylate is an organic compound, the use of heat in drying it would cause it to hydrolyze and decompose. Pavia et al. state that the use of drying agents offers safe and better way of removing water from chemicals while preserving their integrity (376). The purpose of anhydrous sodium sulfate is to dry methyl salicylate, the product obtained from the esterification reaction. Anhydrous sodium sulfate is a solid drying agent that has the ability to absorb water from both solids and solutions. Given that anhydrous sodium sulfate is very dry, it absorbs water and moisture from their environment and become hydrated salts. For anhydrous salts to become hydrated, they absorb a lot of water and moisture; hence, bringing about a drying effect on substances around them. Therefore, anhydrous sodium sulfate removed a lot of water from methyl salicylate making it become dry. The following is the chemical equation showing the conversion of anhydrous sodium chloride into hydrated sodium chloride during the drying process.
Na2SO4(l) + 10H2O (I) → Na2SO4.10H2O (s)
In the experiment, gravity filtration was used in filtering the dried organic layer. Gravity filtration is the basic form of filtration that relies on gravity as the force that determines the rate at which the fluid passes through the filter paper. The main objective of the gravity filtration is to remove impurities from a given solution. In this case, gravity filtration removed particles of the drying agent, which remained in the filter paper as residue, and the collection of methyl salicylate as filtrate.
The solvent in the organic phase was evaporated using the rotary evaporator to obtain methyl salicylic acid. A rotary evaporator is an important apparatus in chemistry that hastens evaporation and the removal of solvent from a solution (Pavia et al. 373). The rotary evaporator has a heating chamber that heats solution for the solvent to evaporate. Moreover, the rotary evaporator has a vacuum suction, which sucks vapor formed and removes them from the flask. Since methyl salicylate is in the organic phase of the solution, evaporation of organic solvent using rotary evaporator enhanced its isolation. To avoid the loss of methyl salicylate, rotary evaporator was stopped when the solvent was apparently finished.
The IR and NMR analyses were used to confirm the formation of methyl salicylate from the reaction of salicylic acid and methanol. Comparison of the spectra of the reactants and products indicates that they have considerable changes, which reflect the formation of methyl salicylate. The reactant (salicylic acid) has a broad O-H stretch of carboxylic acid at 2848.69 cm-1 and 2591.42 cm-1. Moreover, the reactant (methanol) has an O-H stretch at 3233.76 cm-1 as shown in Figure 2. In comparison with the IR of the reactant, the IR spectra of the reactant do not have a broad O-H stretch of carboxylic acid (salicylic acid) at 2591.42 cm-1. However, the IR spectra of the product have O-H stretch at 3180.62 cm-1 and 2954.77 cm-1, which means that it has traces of carboxylic acid and methanol. Furthermore, the IR of the product has a narrow C=O stretch of ester (methyl salicylate) at 1673.77 cm-1 (Figure 3). Therefore, analysis of the IR spectra confirms that salicylic acid and methanol reacted to form methyl salicylate.
The NMR analysis reveals the existence of chemical shifts that reflect the ones of methyl salicylate. The NMR spectra have four peaks, namely, 0.98, 2.15, 2.13, and 3.0. At 10.4 PPM there is singlet peak of 0.98 representing a proton on the –OH group. Additionally, the NMR spectra have two peaks between 6-8 PPM, 2.15 and 2.13, which are doublet peaks representing protons around the aromatic ring of methyl salicylate. At PPM 4, there is singlet peak of 3.0 representing the three protons of the methyl group of the ester.
Determination of the yield shows that the actual yield constituted 66.03% of the theoretical yield. The low yield of the product implies that there were some errors in the experimental process that led to the inefficient production of methyl salicylate. The first possible source of error is that all salicylic acid might not have reacted with methanol. The second possible source of error is that heating under reflux might have caused some of the product to evaporate. As the experiment comprised a series of washing, the third possible source of error is that some of the product might have remained in the aqueous phase.
Works Cited
Chemical Book: Product Chemical Properties 2008. Web.
Pavia, Donal, George Kriz, Gary Lampman, and Randall Engel. A Microscale Approach to Organic Laboratory Techniques. New York: Cengage Learning, 2013. Print.