By definition, photosynthesis is a process whereby light energy (of blue and red wavelength) is converted to chemical energy in the presence of CO2 and water. This process occurs in plants and green algae which eventuate in sugar formation. Basically, the site of photosynthesis in plants is at the chloroplast which contains chlorophyll (apparently green pigment) vital in absorbing light energy.
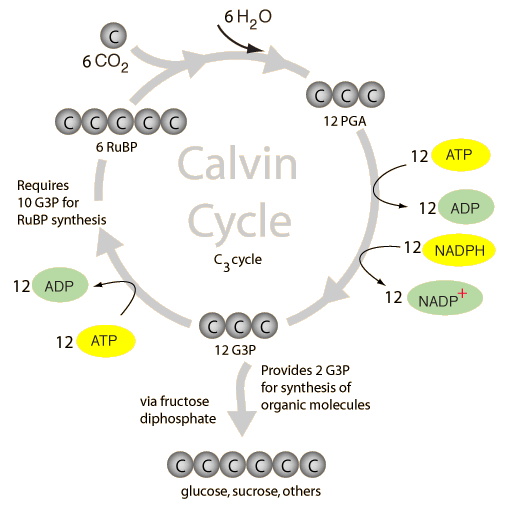
Primarily, the chemical reaction that happens in the chlorophyll is typified by the equation: 6CO2 + 6H2O + light energy → C6H12O6 + 6O2. However, this process is more multifaceted than what meets the eye for it involves two processes: the light and dark reactions. The light reaction “happens at the thylakoid membrane, and it serves to convert light into chemical energy” (Audesirk & Byers, 2008). This energy is then passed onto central chlorophyll where photosynthesis happens to store the energy as ATP (adenosine triphosphate). The dark reaction, which happens devoid of light in the stroma, utilizes CO2 and ATP to form sugars (glucose) in what is referred to as the Calvin Cycle (Fig. 1).
To utilize the sugars processed by plants, animals and plants alike initiate respiration processes which can either be aerobic or anaerobic as manifested in most microorganisms. However, the scope of this paper limits itself to aerobic respiration; a process whereby glucose/organic substrate is broken down in presence of oxygen to release energy, and molecules of water and CO2. The energy is stored chemically as ATP and hence, can be used by other cells within an animal/plant. Basically, in a simplified equation the below reaction occurs:
C6H12O6 + 6O2 → 6CO2 + 6H2O + Energy
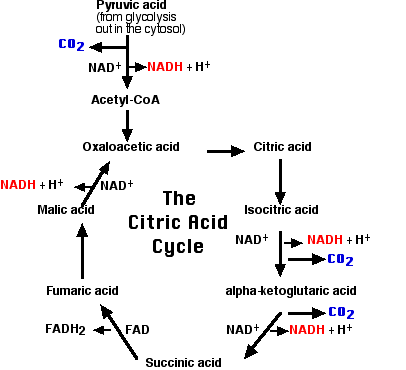
Akin to photosynthesis, respiration is a complex phenomenon that involves two processes: glycolysis which eventuates in the formation of pyruvic acid from glucose, and a process of oxidizing the product to CO2, NADH, and H2O (Fig. 2). Nevertheless, it is the NADH molecules that are utilized in an Electron Transport Chain, and chemiosmosis process to yield ATP molecules. Of note, this process occurs in the mitochondrion of a cell.
As typified by the two processes, each cycle is dependent on the other. As such, the plants and animals are symbiotic thus they accomplish what is known as the Carbon Cycle. To this end, the CO2 is recycled in the system.
Anaerobic and aerobic respiration are different in that the former occurs in the absence of oxygen. Chiefly, this process is analogous to fermentation which results in the formation of either lactic acid or ethanol. To this end, there exist two types of fermentation: alcoholic (Eq. 1) and lactate (Eq. 2) fermentation. These processes are dwarfed by aerobic respiration concerning energy production. They result in the formation of two ATP molecules as opposed to 6 generated from a molecule of glucose through the aerobic process. This process is common in anoxic habitats.
C6H12O6 -> 2CO2 + 2CH3-CH2-OH (ethanol) + Energy (1)
C6H12O6 -> 2C3H6O3 (lactic acid) + Energy (2)
The main chemical pathway of this kind of respiration is “glycolysis, which divides a molecule of the simple sugar glucose into two molecules of pyruvic acid, producing two molecules of ATP in the process” (Audesirk & Byers, 2008).
An enzyme is simply a catalyst in chemical terminology. As such, they act to speed up reactions that would have otherwise happened slowly. Principally, enzyme-substrate interaction is a stepwise cycle that follows the below steps:
- Step 1: Enzyme + substrate.
- Step 2: Enzyme-substrate complex.
- Step 3: Enzyme + product.
To regulate enzymatic reactions, enzyme inhibitors come in handy. Inhibitors would either block the bonding typified by step 1 by binding the substrate (competitive inhibitors), or they would bind on the enzyme changing its active sites lacking a perfect substrate march (noncompetitive inhibitor). Also, the enzyme’s activity could be regulated by the cell through non-covalent modifiers, “which causes a conformational change in between more and less active states of the enzyme” (Audesirk & Byers, 2008). Moreover, this can also be controlled by the cells at the level of biosynthesis.
References
Audesirk, T., & Byers, B. (2008). Biology – Life on earth with physiology (8th Ed.). San Francisco, CA: Benjamin Cummings.