Introduction
Iron (II) compounds which are octahedral in shape which may also transit a spin state crossover have imperative uses in biomedical applications since they detect the onset of two important physical conditions namely temperature and pressure. Besides, they act as visual components in devices that work through display characteristics. Furthermore, iron (II) complexes with the orientation of FeN6 are more considered as far these applications are concerned. It should also be understood that the chemical analysis of unique coordination numbers among the complexes which fall under transition metals has elicited much interest owing to their importance in catalysis. This paper will analyse the crossover complexes of iron coordination compounds in terms of their synthesis, structures and magnetic characterization.
Background
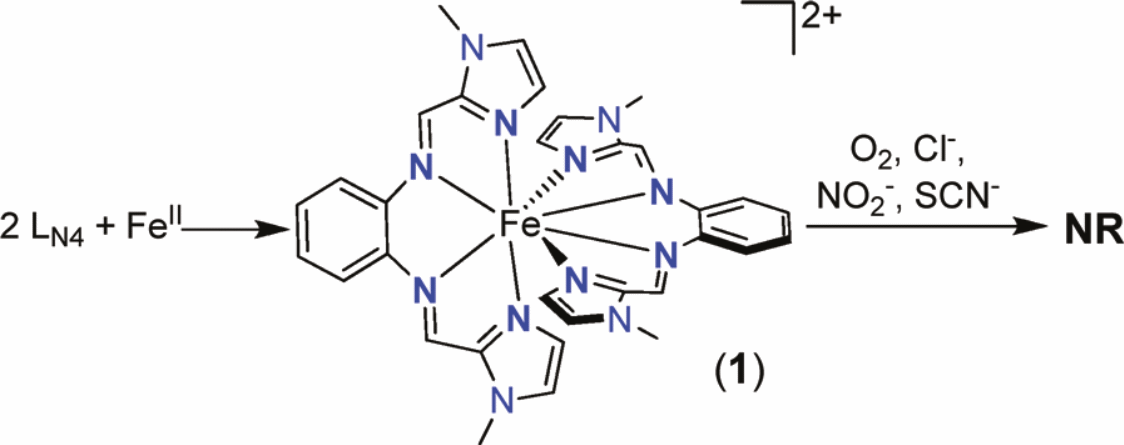
Dominant practical theory in inorganic chemical analysis indicates that coordination numbers from four to six are traditionally assigned for transition metals within the first row. On the other hand, coordination numbers namely three, seven and eight are not common and hardly seen. Moreover, the coordination number of eight is most minimal in occurrence compared to others which are relatively abundant. Although the CNs of eight prove to be rare, there is an increasing quest for the study of their composition due to the high possibility of being used in metallobiomolecules (Trofimenko 126). This is of remarkable biological importance bearing in mind that catalysis has a wide array of applications in this field. The synthesis of this rare species will greatly rely on ligands. Unfortunately, there has not been an orderly study of this group of complexes and therefore no platform for the study of other classified materials. In the synthesis process of the deep-blue 8C iron (II) complex with the chemical formula [Fe (LN4)2] (BF4)2, one of the conditions posed was a complete lack of oxygen besides using the correct proportions of individual reactants.
Structures (X-Ray crystallography)
In order to qualitatively determine the structural orientation of some of these complexes, X-ray structural experiments were conducted. X-ray crystallography can be carried out at different temperatures. At 150 K, Fe [HB (3, 4, 5-Me3pz) 3]2 complex structure proved to be a high spin. The iron- nitrogen single bond interaction has a span of 2.19 Å. (Reger et al. 8865). The angles are equally disorientated in a trigonal manner mostly due to pressure exerted by the rings. Moreover, the geometry of the nitrogen-iron single bonds is measured at 86.2o.
The structure of Fe [B (3-cyPrpz) 4]2 exhibits two molecules which are not related to each other. This is especially so when an x-ray is run at two temperature intervals of two hundred and ninety-four and ninety Kelvin. The mean linear distance of the nitrogen-iron bond at a temperature of two hundred and ninety-four Kelvin is 2.17 Å. The average distances for the other two complexes stood at 2.19 Å and 2.17 Å. This is another vivid demonstration that they both exist at high spin state (Nishio, Hirota & Umezawa 184). In the reverse process of cooling to a temperature of ninety Kelvin, there is an associated change from high spin to low spin while the Fe-N linear bond length reduces by about 0.17 Å. This is usually common with the iron (II) complex. The other iron site is, however, not affected by a temperature drop. Fe [HB (3, 4, 5-Me3pz) 3]2 has a molecular structure that is considered to be super with a 2D flat surface. The red lines in the structure below represent the methyl…pi bond. Each of the molecules has two donors and also two acceptors which are in a 2D structure. In Fe [B (3-cyPrpz) 4]2â (CH3OH), there are three groups consisting of bonds where there is no equal sharing of electrons.
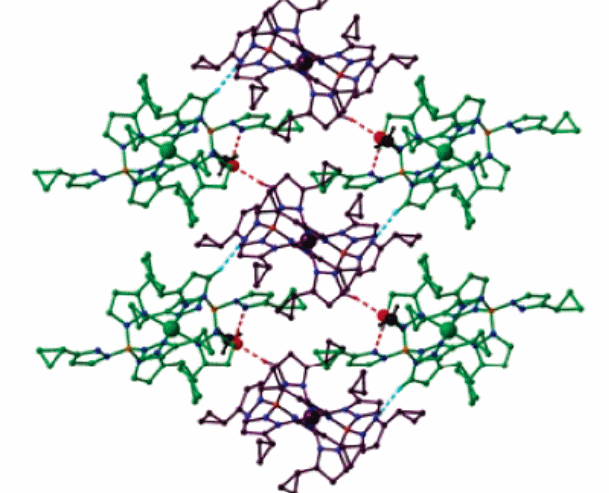
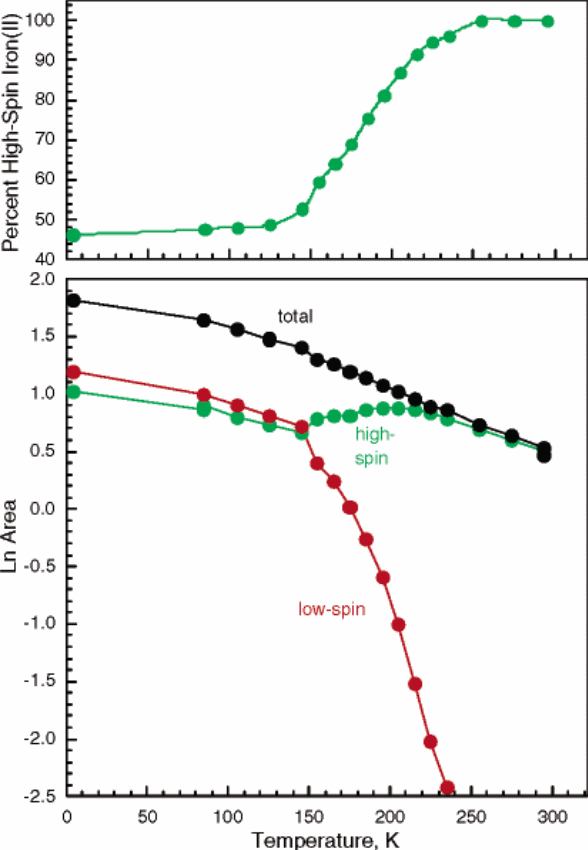
In this case, CH…N, NH…O and CH…O associate in a way that the two crystallographic individual molecules containing both centers of iron into a 2D flat surface (Reger et al. 8867). This occurs when the temperature level is at ninety and two hundred and ninety-four Kelvin structures. The molecule which aptly corresponds to this feature is Fe [B (3-cyPrpz) 4]2 (CH3OH) as in figure 2 below. It should be noted that the green lines in the second figure represent the low spin state while the violet lines represent the high spin states. These supermolecular structures are obtained when the temperature is maintained at 90 Kelvin. It also puts more stress on how X-ray crystallography. Another area of importance in regard to low and high spin states is the effect of temperature. Besides, a complex like Fe [(p-HC2C6H4) B (3-Mepz) 3]2, disruption of ligands may have a significant effect on the nature of the spin state whether high or low. The iron (II) poly-(pyrazolyl) borate structures, for instance, have five unique types of geometric angle changes. Figure 3 shows how temperature influences the nature of the spin state of iron (II) or iron (III). On the same note, the size of the metal ion will tend to increase as the degree of ligand distortions is enhanced. For the sake of the complexes discussed here, the iron (II) complex is more favorable for coordination at low spin.
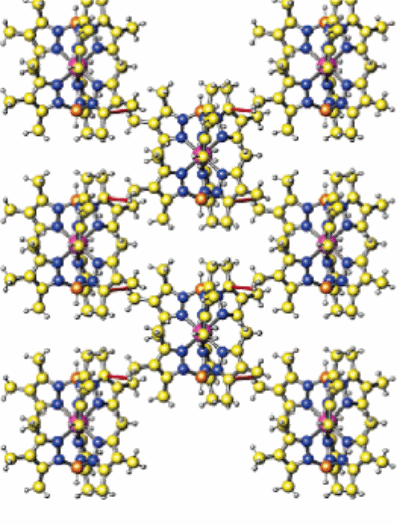
Figure 1 (Reger et al. 8866).
Synthesis
In the synthesis of 8C iron (II) complex [Fe (LN4)2](BF4)2, a mixture of [Fe(H2O)6](BF4)2 and MeCN containing LN4 are reacted in the same vessel. To obtain an optimum yield of eighty-five per cent, the MeCN solution should be twice as much compared to the quantity of the hydrated iron compound used. Results from x-ray pictures indicate that when a ratio of 1:1 is used, a state of balance is achieved (Cotton et al. 94). The equation below shows the manufacture of the complex.
The Fe [HB (3, 4, 5-Me3pz) 3]2 can also be synthesized theoretically. Later, it is also possible to grow the structures to be used for x-ray studies. In its preparation, dichloromethane was reacted with methanol then the solvents were let to evaporate at a moderate rate for a period of not less than forty-eight hours. Other similar iron coordinated which can be synthesized in a more or less the same way are Fe [HB (3cyPrpz) 3]2, Fe [B (3-cy-Prpz) 4]2, and Fe [HB (3-Mepz) 3]2 (Reger et al. 8864). In Fe [HB (3cyPrpz) 3]2, has the ability to turn into crystals .in its synthesis, the complex is more stable on a two-way axis which can undergo easy rotation. Atoms that were not hydrogen are further isolated by the displacement method. In Fe [B (3-cy-Prpz) 4]2. (CH3OH), data is gathered on a transparent piece of material, preferably of crystalline nature. During the collection of data, the temperature is maintained at 294 K (Reger et al. 8864). The temperature of the crystal is then dropped to facilitate cooling. Gradual cooling does not interfere with the orientation of the final crystal formed.
Discussion
When compared to other iron complexes, the above-synthesized product is only stable in the presence of oxygen. In addition, the species does not generate complexes with high oxidation numbers and it does not react with the common negatively-charged ligands like chlorides, nitrites among others. This outcome further demonstrates that the 8C structure attempts to attain more stability amid the competitive state. This characteristic could easily lead to structures with lower coordination abilities. In some cases, the entire coordination structure may break down.
The rigidity of the eight coordination structures is more likely due to the natural orientation of the LN4 ligand. There is a higher likelihood that the chelate rings which are usually grouped into five generated by the above ligand favor quick bond formation with the positively charged species. Eventually, long outstretched bonds are formed with iron and nitrogen coordinated in the bonding process. These long bonds result in the formation of 8C geometry. Additionally, the stability of the above complex can also negate the instability of the associated 8C complex.
Another likely stable complex is that of 8C produced from iron with oxidation numbers of either two or three. They generally tend to maintain their 8C orientation and shape even when in solution. Being a polar complex, the above-synthesized product dissolves in like molecules that are equally organic in nature due to enhanced interaction of bonds which are easily broken during dissolution (Patra et al. 2033). The complex can also actively participate in reduction and oxidation reactions which is coupled with the inter-conversion of iron (III) to iron (II) and vice versa. This reversible electrochemical reaction also proposes that the coordinated compounds and especially the activated complex [FeIII (LN4)2]3+ should be separable.
The chemical analysis involving crystallography did yield comprehensive deductions. In this case, the coordination orientation of FeN8 near the central location of iron depicted some form of distortions. The Fe [HB (3, 4, 5-Me3pz) 3]2 complex has proved to be a high spin type at varying heat levels. Its structural chemistry remains to be unknown. When a careful analysis of supermolecular structures of the complexes discussed above is carried out, it is found out that there Is a negligible relationship between the structural bonding forces and coordination characteristics. In considering the structure of a pyrazolyl ring, it is found that the complex tends to remain at a high spin with a drop-in temperature. This is a vivid demonstration that the complex has numerous internal ring interruptions which may not be easily noted when compared with complexes that do display structural coordination at relatively low temperatures.
Conclusion
There is quite a large array of iron (II) complexes that equally have different derivatives. Additionally, there are several factors that influence the crossover characteristics of this category of complexes as can be observed from experimental results obtained in their study. The most important factor is the congestion of the steric structure which is influenced by the replacement of steric rings. This eventually results in overstretched iron-hydrogen bond. The other characteristic affecting the high or low spin state of coordinated iron (II) compounds is the existence of other branches apart from the hydrogen atom. Structural results reveal that the iron-nitrogen bond distances differ depending on the spin-state level.
Works Cited
Cotton, F. A.; Wilkinson, G.; Murillo, C. A.; Bochmann, M. Advanced Inorganic Chemistry, 6th ed.; New York: Wiley, 1999.
Nishio, M.; Hirota, M.; Umezawa, Y. The CH-ð Interaction: EVidence, Nature, and Consequences; New York: Wiley, 1998.
Patra K Ashis, Dube S Koustubh, Papaefthymiou C Georgia, Conradie Jeanet Ghosh Abhik and Harrop C Todd. Inorganic Chemistry. Stable Eight-Coordinate Iron (III/II) Complexes, 49.5 (2010): 2032–2034.
Reger L. Daniel, Gardinier R. James, Elgin J. Derek, and Smith Mark. Inorganic Chemistry. Structure-Function Correlations in Iron (II) Tris (pyrazolyl) borate Spin-State Crossover Complexes, 45.22(2006): 8862-8875.
Trofimenko, S. Scorpionates: The Coordination Chemistry of Polypyrazolylborate Ligands; London: Imperial College Press, 1999.