Introduction
Substances are always identified based on their physical and chemical properties. One of such physical characteristics is the amount of energy that will be absorbed by a substance per gram—so-called specific heat capacity (Cp).
Cp refers to the amount of heat required to raise the temperature a unit mass (i.e., 1kg) of a substance by 1.00◦C (Dessouki, 2022). As used mostly in metallic objects, Cp form the basis of comparing how metals absorb and transfer heat. The measurement of Cp in the laboratory employs a calorimeter of some kind. Such calorimeter is insulated to reduce heat loss and gain to and from the surrounding, respectively. Assuming no heat gain or loss to the surrounding, the sum of heat lost by the boiling water and the calorimeter is equivalent to heat gained by the solid or metal (He et al., 2022), illustrated in equation (2). The quantity of energy (Q) gained by an object depends on its mass (m) and Cp, as expressed in equation (1).
Were,
In our experiment, the object gains heat from the calorimeter and water. Therefore, heat lost by the calorimeter and water sums to the total heat gained by the object, whose Cp is to be determined.
Where, subscripts in equation (2), including w, c, and s represent water, calorimeter, and solid respectively. Equation (2) can be expressed in terms of specific heat capacities as in equation (3).
Procedure
- The mass of the solid metal cylinder was measured and recorded.
- The dipper of the steam generator was filled with water 2/3 full. The metal cylinder was tied and lowered in the dipper before the investigator heated it till a constant temperature was reached in 8 min.
- The mass of the calorimeter was measured and recorded (i.e., this mass included inner metal cup and stirrer, but not insulating ring).
- A quantity of room temperature water was added to the calorimeter and weighed again.
- The calorimeter was placed in the insulated jacket.
- The investigator measured the temperature of the water in the calorimeter.
- The temperature of the water inside the dipper was measured.
- The heated metal cylinder was rapidly transferred from the dipper to the calorimeter.
- The researcher measured the temperature of water in the calorimeter while stirring. The highest temperature of water was measured and recorded
- Steps 1-9 were repeated for the second metal cylinder.
Table 1: Materials description and quantities
Results and Data Analysis
Using the procedure and materials presented above, the results in Tables 2 and 3 were obtained from the experiment. Signed handwritten data is attached in the figure below.

Table 2: Measurements obtained using the first metal cylinder.
Table 3: Measurements obtained using the second cylinder
Uncertainties
Regardless of accuracy and precision, all measurements have certain degree of uncertainties. Throughout the experiments, such uncertainties were witnessed when measuring two variables, namely mass and temperature. When obtaining the measurement of mass and temperature, the least unit is 0.1. Therefore, uncertainties lied in reading a half of the least unit. Simply put, it was impossible to read 0.05 units from the measuring weighing balance and thermometer.
Calculations
Cp for the metal cylinders can be obtained by substituting the data in Tables 2 and 3 in equation (3), as expressed below. In the calculation, every variable is in SI unit, and the calculation considered uncertainties to obtain maximum and minimum values of the Cp.
Metal cylinder 1
Maximum
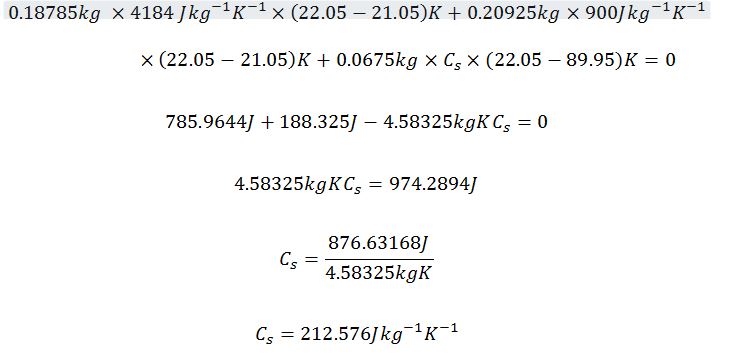
Actual
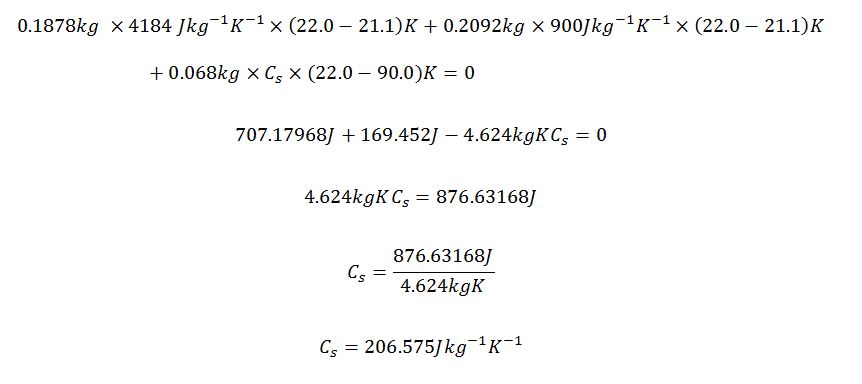
Minimum
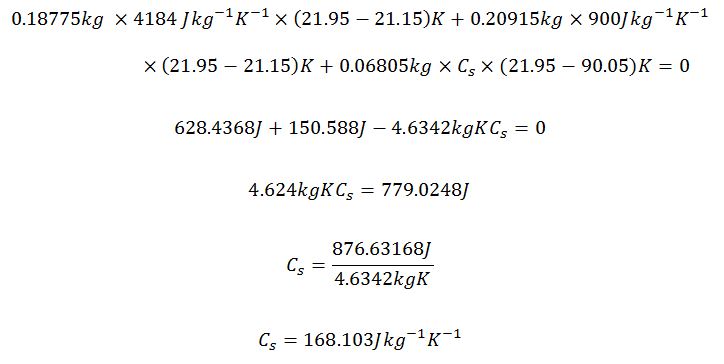
Metal Cylinder 2
Maximum
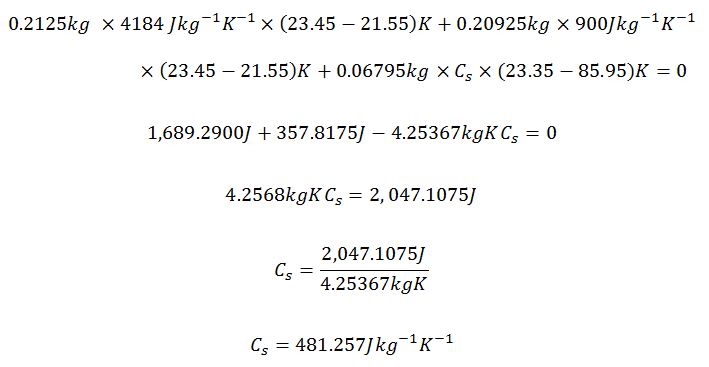
Actual
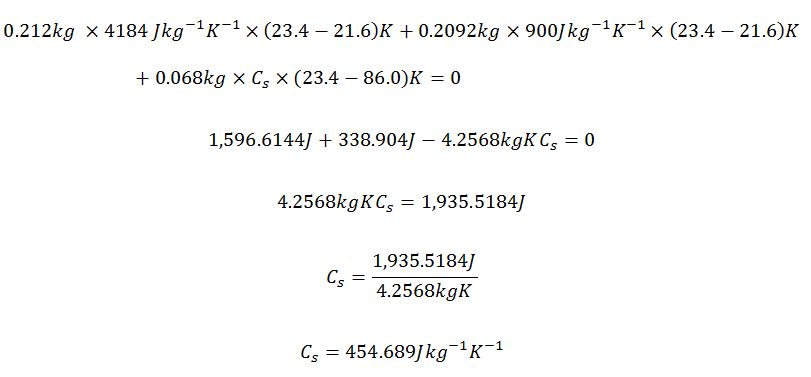
Minimum

Questions
Question (Q11) sought the uncertainties involved in obtaining measurements of mass and temperature. As observed from the measuring instrument the least unit is 0.1 unit. The uncertainty lies in accurately reading the value between. For example, in temperature reading, the investigator could not accurately read the value between ![]() . Similarly, for mass, the value between 68.0g and 68.1g could not be read accurately. In responding to question (12), the specific heat capacities for the metallic cylinders are calculated above. For metal cylinder one, its specific heat capacity ranges between
. Similarly, for mass, the value between 68.0g and 68.1g could not be read accurately. In responding to question (12), the specific heat capacities for the metallic cylinders are calculated above. For metal cylinder one, its specific heat capacity ranges between ![]() and
and ![]() , whereas that of metal cylinder2 is in the range of
, whereas that of metal cylinder2 is in the range of ![]() to
to ![]() . As required in question (Q13), specific heat capacity of water and aluminum calorimeter are
. As required in question (Q13), specific heat capacity of water and aluminum calorimeter are ![]() and
and ![]() , respectively.
, respectively.
Conclusion
The specific heat capacities for metallic cylinders 1 and 2 are in the range of ![]() to
to ![]() and
and![]() to
to ![]() , respectively. These values are obtained in a range to account of uncertainties witnessed in measurement using thermometer and weighing balance.
, respectively. These values are obtained in a range to account of uncertainties witnessed in measurement using thermometer and weighing balance.
References
Dessouki, Y. (2022). Experimental determination of the specific heat capacity of a metal. Web.
He, J., Youssef, R., Hosen, M. S., Akbarzadeh, M., Van Mierlo, J., & Berecibar, M. (2022). A novel methodology to determine the specific heat capacity of lithium-ion batteries. Journal of Power Sources, 520, 230869. Web.