Introduction
Abnormal immune responses to proteins found in certain foods can cause allergic reactions when such foods are ingested by susceptible individuals. Such immune reactions are called food allergies, and the proteins which cause the reactions are called allergens. Foods such as milk, peanuts, eggs, fish, and shellfish are the ones that most commonly cause allergic reactions in the American population. Food allergy affects about 2% of children and about 8% of adults, and cause serious illnesses and in some rare cases even death.
The immune system is made up of cellular and molecular components that can induce and maintenance tolerance to allergens. Various methods have been developed for the diagnosis and management of food allergies. In this paper, the immunological processes of allergic reactions are described giving the relevant anatomical and physiological parameters. Then an overview of pathophysiological features (clinical manifestations) of food allergies is given including their traditional and current diagnostic and management (or treatment) including the immunotherapeutic techniques. Finally, conclusions are given providing a generalized picture of the scope of work still needed to solve the problems of allergies to foods and also to other environmental, domestic, and occupational allergens.
Mechanism of Immune Response to Food Allergies
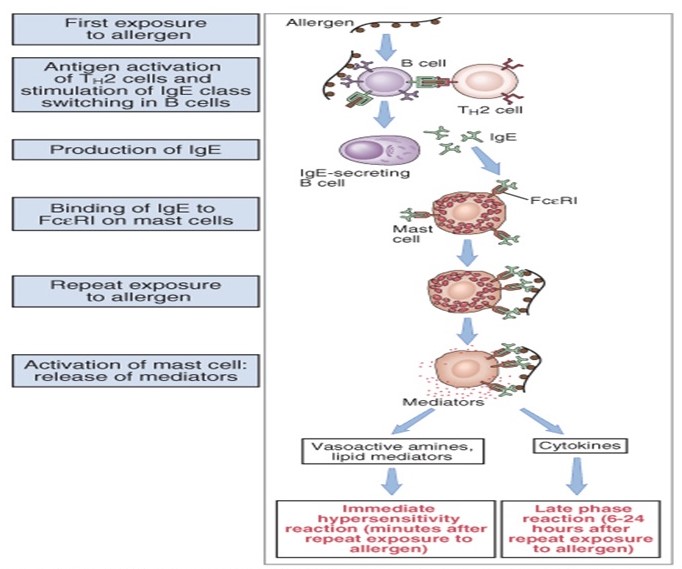
Allergic reactions are usually typed 1 hypersensitivity reactions and involve two arms of the immune system, namely mast cells and immunoglobulin E (IgE). In most cases, when a person ingests food that contains an allergen, the body produces large amounts of IgE specific to food that they had previously been exposed to. The IgE that is released has a high affinity for its receptor on mast cells in the tissues and basophils in the blood, both having mediators of inflammation in their cytoplasm. This process is called mast cell sensitization. On second and subsequent encounter with the same antigen, the IgE and its receptor FcεRI are crosslinked, resulting in the synthesis and release of cytokines and lipid mediators and also the release of granules in the cytoplasm which causes the allergic reaction. Figure 1 shows the sequence of events in immediate hypersensitivity. Immune system cells like T helper 1 and T helper 2 are involved during an allergic reaction to food. The T helper 2 subset is the one that leads to IgE production through B cells activation which involves cytokines such as I-3 and IL-13.
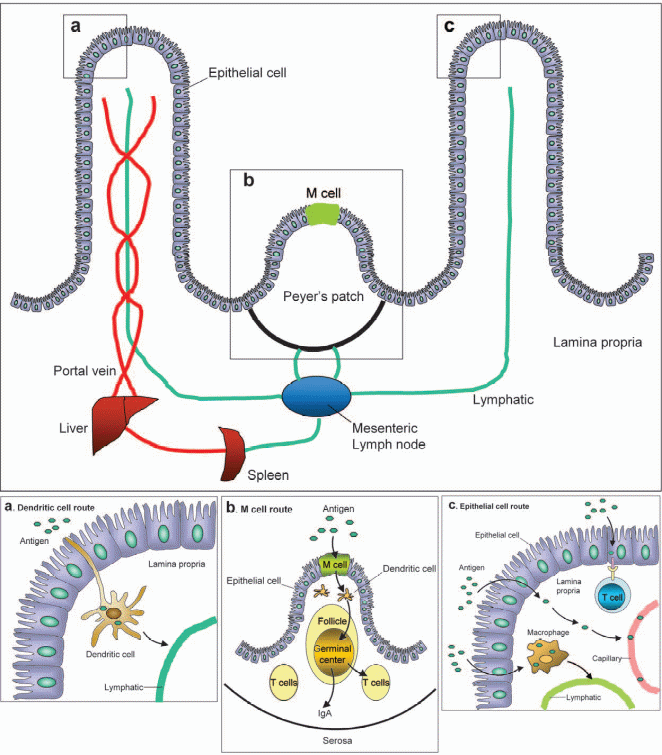
Depending on their structures, proteins or antigens that come into contact with the surface of the intestine are taken up by different cells which can lead to varied responses. The cells that can take up the proteins or the antigens include Peyer’s patches, Microfold cells, and epithelial cells or dendritic cells. Figure 2 shows the antigen uptake sites in the intestine or the gut. After the proteins are taken up by the immune cells, they are broken up or processed into peptides which can then be presented (by antigen-presenting cells, or APCs) to be recognized by immune activation cells. The generation of peptides from intact protein antigens involves modification of the native proteins and is commonly referred to as antigen processing. On the other hand, the display of the peptides at the cell surface by the MHC molecule is referred to as antigen presentation.
As mentioned earlier, the cells that can take up the proteins or the antigens include Peyer’s patches, Microfold cells, and epithelial cells or dendritic cells. These cells are called accessory cells. Other than these accessory cells which can be found in the gut, B-lymphocytes can also internalize or process antigens and present the peptide–MHC complexes in forms that can be recognized by CD4 T cells. Generally, most mammalian cells are capable of endocytosing and processing protein antigens. However, the expression of class II MHC molecules is the critical property that enables a particular cell to function as an antigen-presenting cell (APC).
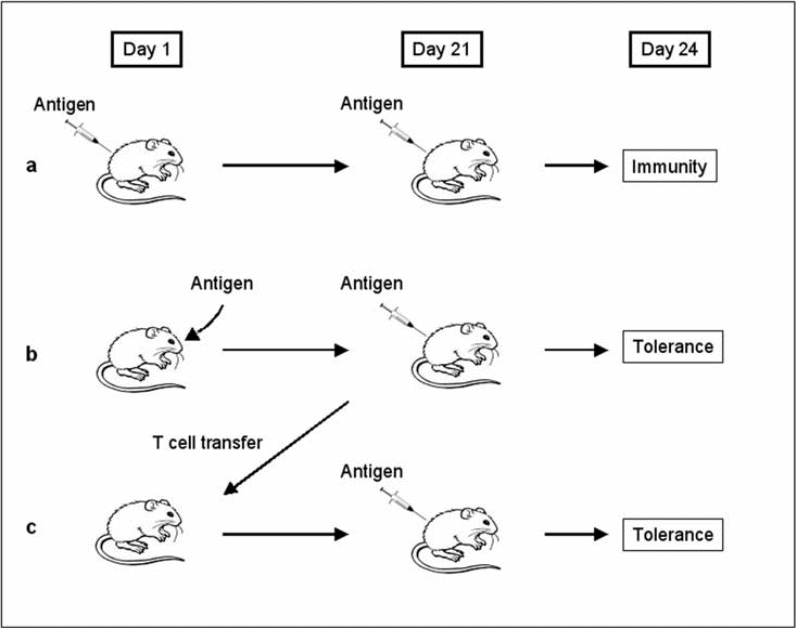
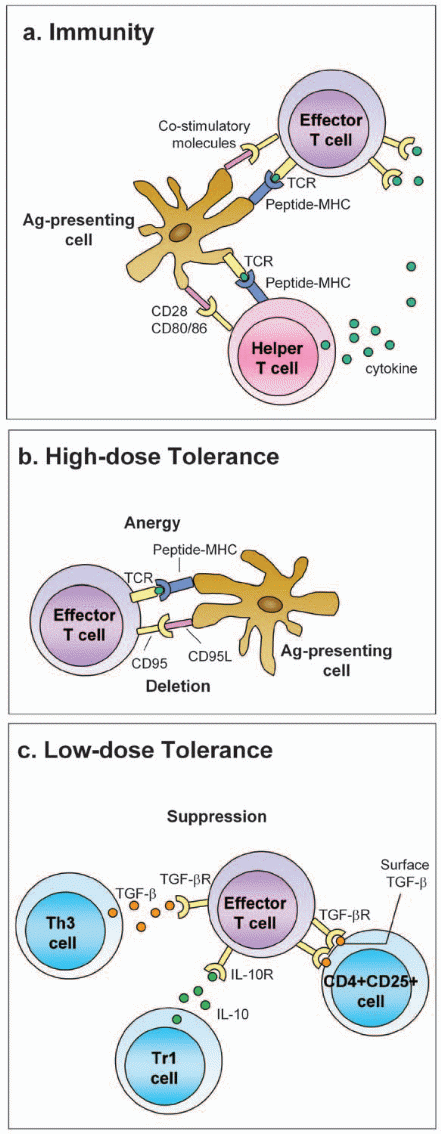
As the antigenic stimulus is activated, immune responses also decline. There are several mechanisms that can inhibit lymphocyte activation. Immunologic tolerance can be induced if an antigen is recognized by specific T lymphocytes when co-stimulatory molecules are absent, e.g. major histocompartibility (MHC) restricted molecule, or by B cells in situations where T cells help are lacking. Immunological tolerance can result from antigen-induced block on maturation and or activation of lymphocytes, or T cells anergy in certain conditions of antigenic exposure or load. Tolerogens are antigens that induce tolerance while immunogens are antigens that generate immune responses.
In review articles, it is generally stated that oral tolerance can be induced by a variety of factors (antigen or host-related) and immune system cells, the most important of which are the regulatory T cells. Food hypersensitivity can result if there are disturbances in the oral tolerance mechanism. Immune responses to foreign antigens are regulated both quantitatively and qualitatively by numerous mechanisms. Factors that influence the induction of specific immunity include the type and amount of antigen, its portal entry, and the participation of accessory cells in the immune responses. These factors may determine which functionally distinct classes of lymphocytes are stimulated, and may influence the balance between lymphocyte activation and tolerance. Figure 3 shows how immunological tolerance can be induced and Figure 4 shows immunological mechanisms of oral tolerance.
Clinical Disorders of Food Allergies
The clinical disorders of food allergies are generally categorized based on interrelated causes some of which can be immunological and others due to the affected organs, or body systems. Common gastrointestinal symptoms ensue from a number of food-induced allergic disorders. These systems are usually differentiated based on diagnostic tests used or sometimes on the kind of illness or syndrome the patient suffers from. Constipation, reflux, and colic are some of the symptoms of the gastrointestinal tract that are associated with allergies to food. In the majority of situations, anaphylaxis usually results from food intake in outpatients, and some reaction outcomes, e.g. after eating nuts, can be fatal especially in young patients with a history of asthma and food allergy. Fatal outcomes easily result if treatment, usually epinephrine administration, is delayed.
Food Allergies Diagnosis
A comprehensive health history combined with a thorough physical examination of the patient is a prerequisite during the clinical diagnosis of an allergy. This will include evaluation of the causative food item, the quantity ingested, allergic reaction time, and other relevant parameters including exercise by the patient, aspirin or alcohol intake, and the reaction consistency. In the majority of cases, food infrequently ingested is usually the cause of an acute allergic reaction than food that was previously tolerated, and acute symptoms of an allergic reaction can include urticaria occurring immediately after ingestion of a food item. In most cases, a food allergy may not be the causative factor for chronic symptoms such as asthma and urticaria.
Confirmation of a diagnosis can in some cases require invasive testing. However, in most cases, the diagnosis relies on the elimination of diet tests, oral food challenge responses, and food-specific IgE antibody determination. Skin prick tests (SPTs) can be routinely used to provide a rapid method of detecting sensitization for IgE-mediated disorders. However it is not always that a suspected food item will be proved to be the causative agent of the allergy after a positive test response. IgE-mediated allergic response can be appropriately confirmed by negative SPT responses. Clinical history and the pathophysiology of the disease are usually of great importance when making maximum use of the results obtained from tests in allergy cases.
Serum tests can also provide an alternative modality for evaluating IgE-mediated food allergy and help through IgE antibodies determination, and it has been found that clinical allergic reactions do correlate with higher IgE values from the diagnostic assays. It will be important to determine the specific IgE-binding epitopes on allergens because this can increase their diagnostic utility if they can be made commercially available, and more studies are needed in this area to develop better diagnostic methods. But the classical placebo-controlled oral food challenge is still the gold standard test for food allergies in patients.
Management and Immunotherapy of Food Allergies
Treatment for food allergies involves the reduction of inflammation, inhibition of mast cell degranulation, or antagonizing the effects of mast cell mediators. The introduction of small doses of the allergen into the body has been one method that is used in the treatment of food allergies. The allergen is introduced into the body in small but increasing dosages and is thought to inhibit IgE production or increase the production of other Ig subclasses. It has also been suggested that other endogenous, environmental, or dietary factors that promote sensitization of these pathways should be considered as some of them, e.g., dried spices can cause allergies and be an occupational health hazard.
In most cases, childhood allergies do naturally resolve which means that more evaluations will be needed sometimes at an increased cost to the patients. Medications that can ameliorate certain aspects of food-induced allergies already exists and classically, antihistamines have been used for such therapies to help manage symptoms of oral allergy syndromes and even to IgE-mediated skin reactions.
A number of new approaches of immunotherapy including the use of peptides on T cell epitopes that lack IgE-binding activity are being tested for use in treatment regimens of food allergies which could result in safe and specific modes of immunotherapy,which require modern characterization methods of proteins and potential allergens. Molecular therapeutic approaches are already being explored for example use of IFN-gamma encoded by cDNA, or vaccination, or IL-4 employing CpG motifs expressed by DNA animal studies. Such novel immunotherapeutic strategies are promising as effective treatment or management of allergen-induced asthma. Already, some specific genes, together with their polymorphisms, are already being studied for their potential usefulness in atopy-linked inflammatory cells action. However, genetic factors which could be important in remodeling at mucosal surfaces and in tissue repair should be understood first.
Immunotherapy will particularly be important in allergy syndromes that are persistent in patients, and also in those susceptible to food allergies. Another potential mechanism of immunotherapy that has been tested involves the injection of food allergens. However, this method has been found to be unsafe. Another alternative to antigen injection as well as injection with engineered antigen, or ingestion of antigen through the gut, are currently being tried. Engineered peanut protein allergies with modified IgE epitope binding sites have been used for immunizing mice with encouraging results.
Low rates of allergies to peanuts have been reported in countries where children normally eat peanut snacks certified as safe for infants. Desensitization involving giving patients small but increasing antigen doses in controlled environmental settings, followed by administration of regular maximum tolerated doses of antigen, has been induced in patients with food allergies by employing oral and sublingual immunotherapy techniques which are then followed by blinded or open food challenge with placebo or antigen.
Treatment protocols in experimental immunotherapies aimed at inducing tolerance in patients have been found to be safe, and any allergic reactions which have occurred have been managed by the use of epinephrine, steroids, and antihistamines. However, it is suggested that it is still unsafe to try such treatments involving immune tolerance induction in regular clinical practice because of the risk of severe reactions which can occur in patients.
Some immunotherapy strategies have involved the use of anti-IgE preparations. For example, patients with peanut allergies have been sort of treated in controlled studies involving injections with TNX-901 and Omolizumab (Xolair) which are both anti-IgE preparations. Also, a concoction of traditional Chinese herbs has been tried as a non-specific immunotherapy agent with promising results, especially in mice models. In other studies, the use of immunostimulatory sequences, for example, CpG motifs, have been found to reverse IgE-mediated sensitization from ragweed allergy in patients. Immunotherapy of reactions to insect stings and inhalants have also been attempted in animal models and human trials with some progress though understanding of the underlying immunological mechanisms is still needed.
There have been contradictory results about immunotherapy efficacy in many trials. This could be explained by differences in genetic make-ups of people (or animals in case of experimental studies involving animal models), types of allergy (e.g. seasonal), and the nature of allergies, e.g. types of foods eaten or the inhalants, or another environmental allergen. Management of patients with food allergies can only be possible if there is an adequate understanding of hypersensitivity versus tolerance in patients. Understanding of tolerance induction mechanisms and their effectiveness to various allergens, and knowing the best ways to deliver antigens, and whether long-term tolerance versus desensitization will ensue is still needed. Promises for novel immunotherapies for food allergies are currently being tried even before molecular mechanisms of immune tolerance are completely understood.
Engineered proteins that lack IgE-binding sites, engineered chimeric molecules bound to Fcg and allergen, co-administration with adjutants, e.g. heat-killed bacteria and CpG), which promotes T helper 1, and use of small overlapping peptides are some of the immune tolerance promoting immunotherapeutic methods which can avoid IgE binding-activation. Another potential method for delaying or preventing allergic syndromes is dietary manipulation and has been the subject of considerations and review. Exclusive breastfeeding of infants who are at high risk of developing allergic diseases for the first 3-6 months of life and nonuse of soy formula or cow milk supplementation have been found in some studies to prevent the development of allergic syndromes. American Academy of Pediatrics currently recommends that mothers of high-risk infants avoid foods e.g. nuts and seafood when they are still lactating.
Conclusions and Future Prospects
Food allergy is still a serious problem in the world today and maybe with us for some time to come unless concerted efforts in research are made. The problem is that we as humans have to eat and our immune systems must also react to the proteins and other components which get into our bodies. Therefore, the solution is to carry out more studies to understand the nature of immune tolerance and also how allergy to different molecular structures is developed. This requires not only a thorough understanding of immunological mechanisms but also molecular biology and biotechnology.
There is also the clinical aspect of allergic syndromes which must be managed by our clinicians, which also requires efforts by our biomedical scientists to develop modern and accurate diagnostic techniques. Not to be forgotten is the fact that some humans not only develop allergic reactions to food items but also to environmental, domestic, and occupational biotic and abiotic materials including pollen grains, mites, and even to rubber products, etc,which not only requires more research and understanding of the structures of the allergens but also of the underlying immunological mechanisms to develop modern disease management systems including immunotherapies.
References
Sampson, H.A., Update on food allergy. J Allergy Clin Immunol, 2004. 113(5): p. 805-19; quiz 820.
Fogg, M.I. and J.M. Spergel, Management of food allergies. Expert Opin Pharmacother, 2003. 4(7): p. 1025-37.
Chehade M and Mayer L. Current reviews of allergy and clinical immunology; Oral tolerance and its relation to food hypersensitivities; J Allergy Clin Immunol 2005;115:3-12
Untersmayr E, Scholl I, Swoboda I, Beil WJ, Forster-Waldl E, Walter F, et al. Antacid medication inhibits digestion of dietary proteins and causes food allergy: a fish allergy model in Balb/c mice. J Allergy Clin Immunol 2003;112:616-23.
Barone KS, Reilly MR, Flanagan MP, Michael JG. Abrogation of oral tolerance by feeding encapsulated antigen. Cell Immunol 2000;199: 65-72.
Hogan SP, Mishra A, Brandt EB, Foster PS, Rothenberg ME. A critical role for eotaxin in experimental oral antigen-induced eosinophilic gastrointestinal allergy. Proc Natl Acad Sci U S A 2000;97:6681-6.
Chambers SJ, Bertelli E, Winterbone MS, Regoli M, Man AL, Nicoletti C. Adoptive transfer of dendritic cells from allergic mice induces specific immunoglobulin E antibody in naı¨ve recipients in absence of antigen challenge without altering the T helper 1/ T helper 2 balance. Immunology 2004;112:72-9.
Man AL, Bertelli E, Regoli M, Chambers SJ, Nicoletti C. Antigen-specific T cell-mediated apoptosis of dendritic cells is impaired in amouse model of food allergy. J Allergy Clin Immunol 2004;113:965-72.
Sicherer SH, Teuber S. Current approach to the diagnosis and management of adverse reactions to foods. J Allergy Clin Immunol 2004;114:1146-50.
Sporik R, Hill DJ, Hosking CS. Specificity of allergen skin testing in predicting positive open food challenges to milk, egg and peanut in children. Clin Exp Allergy 2000;30:1541-6.
Sampson HA. Utility of food-specific IgE concentrations in predicting symptomatic food allergy. J Allergy Clin Immunol 2001;107:891-6.
Boyano-Martinez T, Garcia-Ara C, Diaz-Pena JM, Martin-Esteban M. Prediction of tolerance on the basis of quantification of egg white specific IgE antibodies in children with egg allergy. J Allergy Clin Immunol 2002;110:304-9.
Garcia-Ara C, Boyano-Martinez T, Diaz-Pena JM, Martin-Munoz F, Reche-Frutos M, Martin-Esteban M. Specific IgE levels in the diagnosis of immediate hypersensitivity to cows’ milk protein in the infant. J Allergy Clin Immunol 2001;107:185-90.
Osterballe M, Bindslev-Jensen C. Threshold levels in food challenge and specific IgE in patients with egg allergy: is there a relationship? J Allergy Clin Immunol 2003;112:196-201.
Celik-Bilgili S, Mehl A, Verstege A, Staden U, Nocon M, Beyer K, et al. The predictive value of specific immunoglobulin E levels in serum for the outcome of oral food challenges. Clin Exp Allergy 2005;35:268-73.
Perry TT, Matsui EC, Kay Conover-Walker M, Wood RA. The relationship of allergen-specific IgE levels and oral food challenge outcome. J Allergy Clin Immunol 2004;114:144-9.
Beyer K. Characterization of allergenic food proteins for improved diagnostic methods. Curr Opin Allergy Clin Immunol 2003;3:189-97.
Shreffler WG, Beyer K, Chu TH, Burks AW, Sampson HA. Microarray immunoassay: association of clinical history, in vitro IgE function, and heterogeneity of allergenic peanut epitopes. J Allergy Clin Immunol 2004;113:776-82.
Spergel JM, Beausoleil JL, Mascarenhas M, Liacouras CA. The use of skin prick tests and patch tests to identify causative foods in eosinophilic esophagitis. J Allergy Clin Immunol 2002;109:363-8.
De Boissieu D, Waguet JC, Dupont C. The atopy patch tests for detection of cow’s milk allergy with digestive symptoms. J Pediatr 2003;142:203-5.
Bindslev-Jensen C, Ballmer-Weber BK, Bengtsson U, Blanco C, Ebner C, Hourihane J, et al. Standardization of food challenges in patients with immediate reactions to foods—position paper from the European Academy of Allergology and Clinical Immunology. Allergy 2004;59: 690-7.
Strait, R.T., S.C. Morris, and F.D. Finkelman, IgG-blocking antibodies inhibit IgE mediated anaphylaxis in vivo through both antigen interception and Fc gamma RIIb cross-linking. J Clin Invest, 2006. 116(3): p. 833-41.
van der Walt A, Lopata AL, Nieuwenhuizen NE, Jeebhay MF. Work-Related Allergy and Asthma in Spice Mill Workers – The Impact of Processing Dried Spices on IgE Reactivity Patterns. Int Arch Allergy Immunol. 2010;152(3):271-278.
Perry TT, Matsui EC, Conover-Walker MK, Wood RA. Risk of oral food challenges. J Allergy Clin Immunol 2004;114:1164-8.
Shek LP, Soderstrom L, Ahlstedt S, Beyer K, Sampson HA. Determinationof food specific IgE levels over time can predict the development of tolerance in cow’s milk and hen’s egg allergy. J Allergy Clin Immunol 2004;114:387-91.
Rance F. Novel approaches in treating food allergy using allergens. Nestle Nutr Workshop Ser Pediatr Program. 2009;64:157-63; discussion 164-7, 251-7. Epub.
Abdel Rahman AM, Lopata AL, O’Hehir RE, Robinson JJ, Banoub JH, Helleur RJ. Characterization and de novo sequencing of snow crab tropomyosin enzymatic peptides by both electrospray ionization and matrix-assisted laser desorption ionization QqToF tandem mass spectrometry. J Mass Spectrom. 2010;45(4):372-81.
Takabayashi A, Ihara K, Sasaki Y, Suzuki Y, Nishima S, Izuhara K, Hamasaki N, Hara T: Childhood atopic asthma: positive association with a polymorphism of IL-4 receptor alpha gene but not with that of IL-4 promoter or Fc epsilon receptor 1 beta gene. Exp Clin Immunogenet 2000, 17:63-70.
Oiso N, Fukai K, Ishii M: Interleukin 4 receptor alpha chain polymorphism Gln 551 Arg is associated with adult atopic dermatitis in Japan. Brit J Derm 2000, 142:10043-10046.
Zhu S, Chan-Yeung M, Becker AB, Dimich-Ward H, Ferguson AC, Manfreda J, Watson WT, Pare PD, Sandford AJ: Polymorphisms of the IL-4, TNF alpha, and Fc alpha R1 beta genes and the risk of allergic disease in at-risk infants. Am J Resp Crit Care Med 2000, 161:1655-1659.
Li XM, Srivastava K, Grishin A, Huang CK, Schofield B, Burks W, et al. Persistent protective effect of heat-killed Escherichia coli producing ‘‘engineered,’’ recombinant peanut proteins in a murine model of peanut allergy. J Allergy Clin Immunol 2003;112:159-67.
Levy Y, Broides A, Segal N, Danon YL. Peanut and tree nut allergy in children: role of peanut snacks in Israel? Allergy 2003;58:1206-7.
Enrique E, Pineda F, Malek T, Bartra J, Basagan˜a M, Tella R, et al. Sublingual immunotherapy for hazelnut food allergy: a randomized, double-blind, placebocontrolled study with a standardized hazelnut extract. J Allergy Clin Immunol 2005;116:1073-9.
Patriarca G, Nucera E, Roncallo C, Pollastrini E, Bartolozzi F, De Pasquale T, et al. Oral desensitizing treatment in food allergy: clinical and immunological results. Aliment Pharmacol Ther 2003;17:459-65.
Meglio P, Bartone E, Plantamura M, Arabito E, Giampietro PG. A protocol for oral desensitization in children with IgE-mediated cow’s milk allergy. Allergy 2004;59: 980-7.
Buchanan AD, Green TD, Jones SM, Scurlock AM, Christie L, Althage K, et al. Egg oral immunotherapy in nonanaphylactic children with egg allergy. J Allergy Clin Immunol 2007;119:199-205.
Staden U, Rolinck-Werninghaus C, Brewe F, Wahn U, Niggemann B, Beyer K. Specific oral tolerance induction in food allergy in children: efficacy and clinical patterns of reaction. Allergy 2007;62:1261-9.
Marshall JD, Abtahi S, Eiden JJ, Tuck S, Milley R, Haycock F, et al. Immunostimulatory sequence DNA linked to the Amb a 1 allergen promotes T(H)1 cytokine expression while downregulating T(H)2 cytokine expression in PBMCs from human patients with ragweed allergy. J Allergy Clin Immunol 2001;108:191-7.
Horner AA, Raz E. Immunostimulatory sequence oligodeoxynucleotide- based vaccination and immunomodulation: two unique but complementary strategies for the treatment of allergic diseases. J Allergy Clin Immunol 2002;110:706-12.
Nowak-Wegrzyn A, Sampson HA. Food allergy therapy. Immunol Allergy Clin North Am 2004;24:705-25.
Frick OL, Teuber SS, Buchanan BB, Morigasaki S, Umetsu DT. Allergen immunotherapy with heat-killed Listeria monocytogenes alleviates peanut and food-induced anaphylaxis in dogs. Allergy 2005;60:243-50.
Zhu D, Kepley CL, Zhang K, Terada T,Yamada T, Saxon A. Achimeric human-cat fusion protein blocks cat-induced allergy. Nat Med 2005;11:446-9.
Muraro A, Dreborg S, Halken S, Host A, Niggemann B, Aalberse R, et al. Dietary prevention of allergic diseases in infants and small children. Part III: critical review of published peer-reviewed observational and interventional studies and final recommendations. Pediatr Allergy Immunol 2004;15:291-307.
American Academy of Pediatrics Committee on Nutrition. Hypoallergenic infant formulas. Pediatrics 2000;106:346-9.
Mabe DO, Singh TS, Bello B, Jeebhay MF, Lopata AL, Wadee AS. Allergenicity of latex rubber products used in South African dental schools. Afr Med J. 2009;99 (9):672-4.
Baatjies R, Meijster T, Lopata A, Sander I, Raulf-Heimsoth M, Heederik D, Jeebhay M. Exposure to flour dust in South African supermarket bakeries: modeling of baseline measurements of an intervention study. Ann Occup Hyg. 2010;54(3):309-18.