Introduction
Human beings take food in order to obtain nutrients and energy. The food includes proteins, carbohydrates, as well as lipids. Proteins are the main structural building blocks of the body that are used in building and repair of worn out tissues. Carbohydrates are the primary source of energy while lipids are involved in a variety of functions that include tissue building, transport and storage. Nutrients are small sub-units obtained from a large mass of ingested food that is continually broken down as the food moves from a mouth to the intestines. This breakdown involves a number of enzymes that are protein in nature, and is generally known as digestion.
This essay is on the process of digestion, absorption and assembly of proteins in cells. The first section discusses how digestion of proteins takes place in the stomach. The second section looks at how proteins are absorbed across the intestinal wall. Before drawing a conclusion, attention is given to the assembly of amino acids in cells during the process of protein formation
Digestion of proteins
Structure and source of proteins
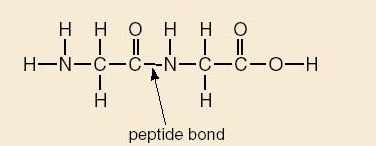
Proteins are essential for life. Proteins are involved in tissue formation in the body, speeding body reactions, transportation of macromolecules, and formation of hormones, to mention just a few. The main sources of proteins are animals and plants. Examples of protein-rich foodstuff include cheese, chicken, bacon, beef, cod, herring, milk, soya flour, peanuts, as well as peas. Structurally, proteins are long chains of sub-units called amino acids linked by peptide bonds. Although there are hundreds of proteins in existence in plants and animals, all of them are derived from 20 different amino acids. These are further classified as essential and non-essential amino acids.
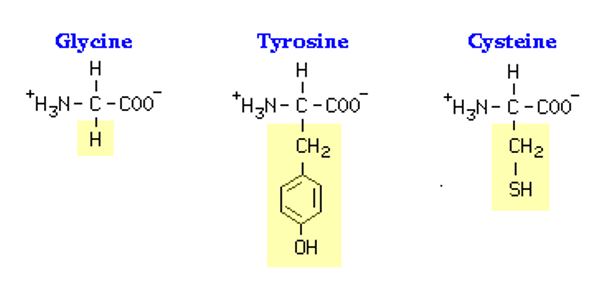
Essential amino acids have to be supplied from diet as the body cannot make them, while non-essential ones do no need to be supplied in diet as the body can make them (Open University). Essential amino acids include lysine (Lys), methionine (Met), threonine (Thr), leucine (Leu), isoleucine (Ile), valine (Val), phenyalanine (Phe), tryptophan (Try), histidine (His),Arginine (Arg), tyrosine (Tyr),as well as cysteine (Cys). Non-essential amino acids are glycine (Gly), alanine (Ala), serine (Ser), proline (Pro), glutamate (Glu), glutamine (Gln), aspartate (Asp) and asparagine (Asn).
Amino acids
Amino acids are the basic building units of proteins. Amino acids are made up of a central carbon bonded to a Hydrogen (+H) ion, a carboxyl group (COO–), an amino group (–NH2) and an all-important side chain(R-group). The R-group is the most important as it determines the chemical properties of an amino acid and ultimately, its interaction with other amino acids in a protein.
Digestion of proteins in the stomach
Digestion of protein starts in the stomach. However, in the mouth, the portion of protein food mass is chewed and reduced into small particles. This is important as it increases the surface area for enzymatic action in the stomach (Othman).
In the stomach, gastric juice (hydrochloric acid) released from the wall of the stomach, initiates the digestion of proteins (Selim). Gastric juice performs among other functions activation of pepsinogen to its active form pepsin and providing a low PH in stomach, a condition suitable for the working of pepsin. Pepsin breaks down the central peptide bond in long proteins resulting in shorter polypeptides that are transferred to the small intestine where the rest of digestion takes place (Open University, n.p; Selim n.p).
Digestion in the small intestine
Digestion of proteins is completed in the small intestines under the action of enzymes from the pancrease and the walls of small intestine itself. By the end of digestion, the long chain protein molecules would have reduced to amino acids and a few tri-and di-peptides chains (Sturm). When broken polypeptides enter the duodenum, they trigger the release of cholesytokinin into the bloodstream (Selim). This action initiates the release of hydrolytic enzymes from the pancrease and peptidases from the intestinal wall (Sturm). Enteropeptidase from the intestinal wall converts pancreatic trypsinogen to trypsin.
Trypsin is responsible for activating more trypsin and proenzymes of chymotrypsin, elastase, and carboxypeptidases A and B (Selim; Sturm). Trypsin hydrolyzes the peptide bonds in small amino acids, such as arginine, lysine and histidine. Chymotrypsin breaks the peptide bonds in aromatic amino acids that is activated by trypsin. Carboxypeptidase hydrolyzes the terminal peptide bond at the carboxyl end of the chain. Elastase acts on the peptide bond of glycine, alanine and serine, and is also activated by trypsin.The final breakdown occurs at the surface of intestinal epithelial cells where endopeptidases and aminopeptidases break down proteins into free amino acids, di- and tripeptides (Sturm).
This marks the end of digestion of proteins. Next, the amino acids are absorbed across the wall of the small instestine and re-joined again to build new proteins.
Absorption of proteins
Sodium and peptide transport systems are involved in the absorption of amino acids. The transport systems require ATP energy, suitable temperature and various transporting agents in order to function properly. There are two major protein transport systems, namely, carrier protein transport system and glutathione transport system. The carrier protein transport system is dependent on sodium. In this system, the co-transporter upon binding sodium binds the amino acid. This leads to alteration of its structure enabling it to pass through the mucosal membrane of intestinal epithelia cells and releasing the amino acid and sodium into the cytoplasm (Bowen). This kind of transport system relies heavily on the electrochemical gradient of sodium across the epithelium in order to function properly (Bowen). Dipeptides and tripeptides are transported by H+ dependent co-transporters (Bowen).
Absorption of protein results in the deposition of amino acids into the cytoplasm. Amino acids are not left to linger in the cytoplasm; they are used to form protein through an interplay of mechanism that involve special RNA (Ribonucleic Acid), enzymes and DNA (Deoxyribonucleic Acid).
Assembly of proteins in the cell
Protein synthesis begins in the nucleus when the cell receives cellular communication to make a certain quantity of a protein depending on the need (Liang and Blohowiak 2). This is followed by the unwinding of DNA molecule. The unwinding exposes a gene that codes for a particular protein.
Nucleotides under the influence of enzymes align along the exposed portion of DNA leading to the formation of a molecule of messenger RNA (mRNA). The DNA and RNA are made up of four nucleotides. The nucleotide bases are Guanine (G), Cytosine (C), Adenine (A) and Tyrosine (T)/Uracil in RNA. After formation, the mRNA migrates to the cytoplasm from the nucleus via nuclear pores.
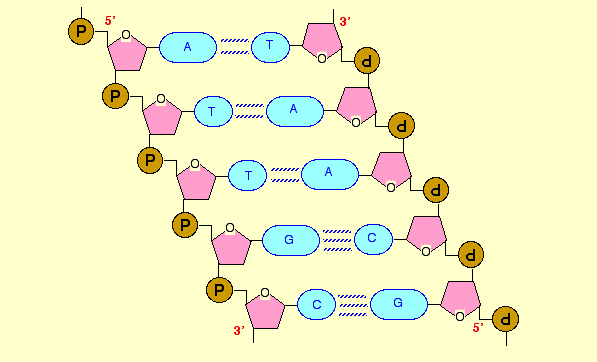
In the cytoplasm ribosomes bind the messenger RNA. The ribosome reads the order of nucleotides on the mRNA three at a time. The arrangement of three successive nucleotides is known as codon. Each codon represents a specific amino acid. When a codon is read, its corresponding amino acid is fetched by a transfer RNA (tRNA), and brought to the ribosome. This reading continues down the mRNA, and each time the correct amino acid is brought to the ribosome by a different tRNA. When the mRNA has been completely read, the tRNA breaks off the mRNA chain, and the amino acids get linked via peptide bonds eventually forming a polypeptide chain. Formation of peptide bonds requires ATP energy. At the end of formation, the polypeptide assumes its conformation, and then it is released. To meet the quantity of protein required, similar copies of the protein are made based on the first ribosome on the mRNA (Liang and Blohowiak 16). When protein synthesis is complete, the mRNA is recycled and the nucleotides diverted to where it is needed (Liang and Blohowiak 17).
Conclusion
Protein digestion, absorption and synthesis involve a series of complex steps a protein diet undergoes from the time it is consumed to an eventual utilization of its sub-units in the manufacture of new protein materials for the body. In the stomach, under the influence of gastric juice, protein is partially broken down into smaller polypeptide chains. The small sub-units are transferred to the small intestine where a host of pancreatic and intestinal enzymes finally complete the breakdown of the polypeptide chain to amino acid, di and tri-peptides. These are then absorbed across intestinal epithelial cell into the bloodstream and thence into the cytoplasm via a set of complex transport systems. Free amino acid, tri-peptide and dipeptides are not left to linger in the cytoplasm but are re-assembled back into long chains of protein. This process starts in the nucleus of the cell and is completed in the cytoplasm. DNA, derivatives of RNA and enzymes are involved in the synthesis of new protein.
References
Bowen, Riley. “Absorption of Amino Acids and Peptides.” Columbia State University. 2006. Web.
Clark, Jim. “DNA Structure.” 2007. Web.
Liang, Barbara and Chad Blohowiak. “Protein Synthesis.” 2012. Web.
Open University. “Nutrition:Proteins.” Learning Space. 2012. Web.
Othman, Rozaini. “Human Digestive System.” 2010.Web.
Selim, Mohammed Ibrahim. “General Protein Metabolism.” 2010. Web.
Sturm, Noel. “Digestion and Absorption of Proteins & Carbohydrates.” 2010.Web.