Introduction
The rapid increase in world population is putting a lot of force on the environment. Water occupies 70% of the earth space and is also the most significant component for human existence. It is also a major component for industrial processes. Currently, households account for 50% of the global water consumption while the other 50% goes to agricultural activities. The need for water increases with an increase in growth population (Tang & Chen 2002).
The textile industry is among the biggest user of water and complex chemicals at various phases of textile processing. As such, the industry creates a risk of environment pollution and other health hazards. In the last few years, the Australian textile industry consumed large volumes of drinking water. As a result of the increased demand for textiles, the levels of wastewater have increased rapidly (Anjaneyulu, Chary & Raj 2005). Wastewater generated from textile industries contains organic dyes and other toxic products which have mutagenic effects on life. The discharge of textile industry wastewater into the environment cause serious health and environmental problems (Türgay et al. 2011).
Wastewater from the textile industry is one of the highest pollutants in the manufacturing sectors (Şen & Demirer 2003). According to past studies, the most common pollutants in the textile wastewater are large amounts of suspended solids (SS), pH, colour and salts. Recalcitrant organics have dye, sizing agents and dying aids. Moreover, “dye is the most difficult constituent of the textile wastewater to treat” (Şen & Demirer 2003). Also, the textile wastewater has high temperature, biochemical oxygen demand (BOD), acidity, high chemical oxygen demand concentration (COD), and other soluble substances (Ahn, Chang & Yoon 1999; Kim et al. 2002). All these contents make the processes of treat textile wastewater more complex. The main objective of this project is to remove dye from textile wastewater.
The figure below shows some harmful direct and indirect effects of textile wastewater (Verma, Dash, Bhunia, 2012)
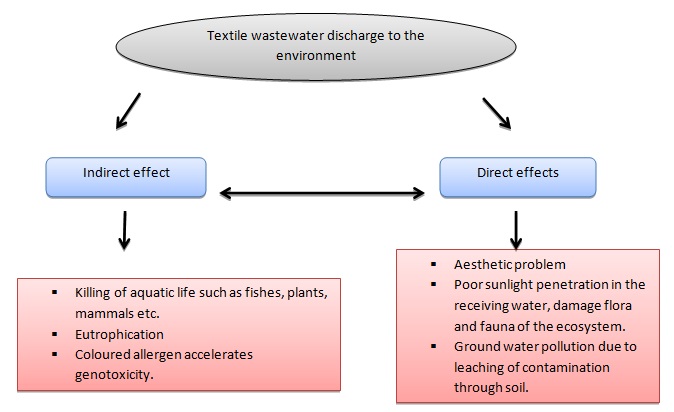
Project definition
Key objective /issue to be investigation
The key objective of this project is to remove dye wastewater through the use of different methods of textile wastewater treatment. The coagulation method is one of the methods used in the treatment of textile wastewater. The purpose of the coagulation method is to reduce COD remove colour from textile wastewater by using several chemical and physical treatment methods (Ramakrishna & Viraraghavan 1997). The other objective is to understand the characteristic of textile wastewater and the influence the removal of dyes after adding different chemicals to it and how it is able to decrease the colour removal of the dyes.
Project benefit
Environmental Benefits
The removal of dye wastewater from the textile wastewater is considered advantageous because most of organic pollutants and other chemicals are neutralized. Additionally, “pollutants are removed and the quality of the water is improved, allowing for final discharge to the environment without significant risk” (Islam, Mahmud, Faruk, & Billah 2011, p. 429). This means that the effluent that is released to the environment is free from any risks on humans and the environment. Additionally, the removal of dye wastewater from the textile wastewater improves the quality of surface groundwater, thus protecting the environment.
Economic Benefits
There are several economic benefits associated with the removal of dye wastewater from the textile wastewater. For example, it leads to economic benefits with references to income, health, and environmental security (Ofoefule, Uzodinma, & Ibeto 2011). Additionally, treated wastewater promotes agriculture which improves food production, encouraging security and income. It also saves resources which makes it economically beneficial.
Social Benefits
From a social perspective point of view, the removal of dye from the textile wastewater improves the value and quality of life. For example, after the wastewater has been treated, people living around these areas are able to utilize clean water for household use, irrigation, and bathing, among other uses. Other than improving the quality of life, the removal of dye from the textile wastewater prevents diseases, contamination, and other health hazards (Islam et al. 2011). Moreover, sanitation is improved which enhances public safety. Lastly, converted biodegradable wastewaters may be used to produce biogas, resulting in cleaner air, and improved sanitary conditions (Ofoefule, Uzodinma, & Ibeto 2011).
Project Deliverable
The research is vital as it provides insights on the social, economic, and environmental benefits associated with the removal of dye wastewater from the textile wastewater. As such, an awareness is created that would improve the quality of life and sanitation. Moreover, the evaluation of different methods used in textile wastewater treatment show the effectiveness of the methods and how they could be applied. By understanding the characteristic of textile wastewater, we are able to define the most effective and efficient method and the influence of coagulants on the removal of dyes after adding different chemicals.
Literature Review/Research
Waste water and water treatment consist of three main categories which are: chemical method, physical method, and biological method. Najafi and Movahed (2009) note that “There are many processes available for wastewater treatment in these industries: chemical oxidation, foam flotation, electrolysis, biodegradation, adsorption, chemical coagulation and photocatalysis” (p. 3053). These three categories are has shown in the diagram below. The main scope of this research is to establish the detailed process of coagulation/fluctuation.
Physical treatment method
Adsorption
Verma et al. (2012) have opined that “adsorption method for color removal is based on the affinity of various dyes for adsorbents” (p. 158). The major chemical and physical factors associated with the method as provided by Anjaneyulu et al. (2005) are contact time, pH, temperature, size of particle, surface area, and rate of interaction. The selection criteria for adsorbent is based on adsorbent regeneration possibilities, target compound capacity, and affinity, level (Karcher et al., 2002). According to Walker and Weatherly (1997), the commonly used adsorbent is activated carbon. Nonetheless, the efficiency is determined by wastewater characteristics and carbon material adopted. The drawbacks of adsorption are such as extra maintenance costs, and threat of disposed adsorbents among others. However, the method is not restricted to colour removal as it is applicable in wastewater and water treatment.
Table 1. Effect of initial dye concentration by diatomite and percentage of dye removal. Source: Al-Ghoutiet al.2003.
All the experimental conditions were the alike during the process although the pH was 11, 3, and 3 for methylene blue, hydrolysed reactive black and yellow, respectively (optimum value). The experimental conditions were:
- Mass of diatomite =0.05 g,
- volume of solution =50 cm3,
- equilibrium time = 48 h,
- Particle size = 106–250 µm,
- temperature =200C, and
- Shaking speed = 125 rpm.
Coagulation –flocculent
This method entails the “the destabilisation of dye solutions using coagulant(s), which can be classified into two main categories namely, metal coagulants and polymers” (Zahrim et al. 2011, p. 4). Therefore, chemicals are added to destabilize particles for flocculation. Flocculation is the process of bringing the particles together so that they aggregate into larger particles (Zahrim et al. 2011). The most used coagulants are Alum: Al2 (SO4)3.14H2O, Aluminium chloride: AlCl3, Ferric chloride: FeCl3, and Ferrous sulphate: FeSO4. The main scope is to determine how these coagulants are used to destabilize particles for flocculation (Karcher, Kornmuller & Jekel 2002). The coagulation relationship is to be described briefly in the thesis.
Previous research
Multiple studies on metal coagulant/polymer for removal of dyes are reviewed. Like in past studies, the experiments were carried out using C.I. Reactive Red 45 (RR 45) and C.I. Reactive Green 8 (RG 8) (Papic et al. 2000). The λmax values and chemical structures are presented below.
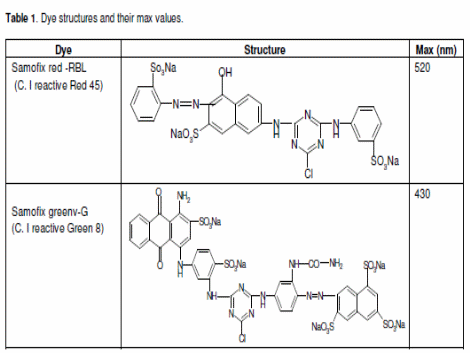
At the absorbance maximum, calibration curves were made for each dye.
Dyes were dissolved into distilled water to form synthetic wastewater of concentration of 1 g /L. Later jar test procedure carried out at various pH conditions (3–5), room temperature (22 to 25 °C) and coagulant dosages (0.4–5.0 g/L). Lum chloride- AlCl3 6H2O of gram molecular weight 241.45 was the coagulant used. Synthetic wastewater was put into 500 ml the coagulant was added and magnetic stirrers used to mix at 300 rpm. To vary the pH, Na2CO3 was added after the coagulant had been added and stirred at a speed of 50 rpm for the next twenty minutes. This was followed by a sedimentation period. After 2 hours, the height of sludge was measured followed by the determination of the dye concentration using SPEKOL 210 MA 9525-Iskra spectrophotometer. After two hours, the height of the precipitate was measured and the percentage of the sludge volume calculated. Coagulation was applied as a major treatment process to remove the high concentration of reactive dyes in wastewaters. Sarasa et al. (1998), combined ozone and chemical coagulation treatment of wastewater to establish effectiveness. The results showed that ozone was ineffective in the removal of azo dyes. However, coagulation method was very effective as it removed all compounds (Najafi & Movahed 2009)
Type of reactive dye: Red 45, Green8. Source: (Papic et al. 2004).
In developed nations, the coagulation–flocculation method is widely used in textile wastewater treatment plants. Gähr et al. (1994) noted that coagulation is applied for pre-treatment, post-treatment, and main treatment system. Past research shows that coagulation not only removes sulphur, it also disperses dyes (Marmagne & Coste, 1996; Vandevivere et al., 1998). However, it is ineffective in acid, direct, reactive and other dyes removal (Vandevivere et al., 1998).
Effect of the coagulant type
Metal coagulant
In their study, Butt et al. (2005) reported that Ferric chloride could remove 79–82 percent of reactive dyes whilst ferrous chloride could only decolourise 58–64 percent. In a separate study, ferric chloride efficiently removed Levafix Brilliant Red ERN although it was not efficient in the removal of Remazol Brilliant Violet 5R (Carvalho et al. 2002). In their study, Kim et al. (2004) studied coagulation using ferric chloride on two reactive dyes. They observed that the maximum removal of COD were 23 percent, 41 percent of dye at pH 7 for Reactive Blue 49, and 63 percent of COD, 44 percent of dye at pH 6 for Reactive Yellow.
Organic polymer coagulant
A major interest has been on the application and synthesis of natural. For example, the removal of an acid dye using chitosan has been studied (Szygula et al.2009). At sedimentation time of two hours and a high concentration of chitosan (91–141 mg/l), the findings showed that the removal of C.I. Acid Blue 92 was about 99 percent. This method has not been studied extensively, despite its numerous advantages. As such, more research is required. New technologies need to be developed to obtain chitosan with precise characteristics to acquire maximum performance. The studies carried out have showed that relatively high concentrations of polymer are still required for dye removal. This makes the cost of treatment more expensive. Conversely, the coagulation is not efficient at lower dosage of polymers. Furthermore, higher dosages could boost the chances of toxicity of discharged water waste and tainting of membranes.
Filtration
Filtration methods are applied in filtration and recycling in the textile industry. It is used in pigment-rich streams as well as in mercerizing and bleaching wastewaters. Porter et al. (1997) study showed that temperature and chemical composition of the wastewater determine the type and porosity of the filter to be applied. However, the method is costly and results to secondary waste which requires further treatment (Robinson et al., 2001).
Reverse osmosis
Reverse osmosis is used in dye house wastewater to enhance decolourization and elimination of chemical auxiliaries (Gaehr, Hermanutz & Oppermann 1994). It allows the removal of chemical auxiliaries, mineral salts, and hydrolyzed reactive dyes. It should be noted that the higher the concentration of dissolved salt, the greater the energy required for the separation process (Ramesh Babu et al., 2007).
The biological treatment
The biological process is more efficient than flocculation. The efficiency is influenced by temperature, oxygen concentration, and the biomass in the oxidation tank. Basically, biomass concentration (between 2500-4500 mg/l) with oxygen of about 2 mg/l is proffered. The biological treatment methods are divided into aerobic and anaerobic treatment. The aerobic biological treatment is widely applied because of its high efficiency (Wang et al. 2011)
Table 1: Summary of the treatability of physico–chemical treatments for inorganic effluent (2006).
Tentative brief timeline
Below are the stages to be followed to achieve the desired outcomes.
Conclusion
Textile dyeing wastewater is one of the major sources of pollution. Wastewater is composed of high content of colour, BOD and COD, among other impurities. Direct discharge of wastewater without treatment poses a threat to human health and the ecological environment. To curb these undesirable effects, many countries have enacted strict emission standards for the textile dye waste water. Waste water and water treatment consist of chemical method, physical method and biological methods. Chemical method entails oxidation, ozonation, and electrolysis while physical method is mad of reverse osmosis, filtration, adsorption, and coagulation/ flocculation. On the other hand, biological method entails the use of biological enzymes and microorganisms. The most effective removal methods are electro-kinetic coagulation and ion exchange coagulation although they generate high sludge properties. Flocculation is the process of bringing particles together so that they aggregate into larger particles
Reflective paper/ document
One of the major sources of ground and surface water pollution in the 21st century is discharge of partly treated wastewater straight into the surface water bodies. From the literature review, we have established that the wastewater treatment technique as used may be inadequate to get rid of all the organic pollutants. By conducting this research, I have realised that there is need to develop new technologies to obtain chitosan with precise characteristics to acquire maximum performance. I have also increased my level of knowledge on the major methods used in wastewaters treatment by to remove dyes and other components. These methods are coagulations, aeration, and filtration. The most effective method of all is coagulation although it is expensive in terms of costs and materials used. I have also learned that direct discharge of wastewater without treatment leads to a series of serious harm to health and ecological environment.
References
Al-Ghouti, MA., Khraisheh, MAM, Allen, SJ & Ahmad, MN 2003, ‘The removal of dyes from textile wastewater: a study of the physical characteristics and adsorption mechanisms of diatomaceous earth’, Journal of Environmental Management, vol. 69, no. 3, pp. 229-238.
Ahn, DH, Chang, WS & Yoon, TI 1999,’ Dyestuff wastewater treatment using chemical oxidation, physical adsorption and fixed bed biofilm process’, Process Biochem. vol. 34, no. 5, pp. 429-439.
Anjaneyulu, Y, Chary, NS & Raj, DSS 2005,’ Decolourization of industrial effluents available methods and emerging technologies-a review’, Reviews in Environmental Science and Biotechnology, vol. 4, no. 4, pp. 245-273.
Butt, MT, Arif, F, Shafique, N & TImtiaz, N 2005,’Spectrophotometric estimation of colour in textile dyeing wastewater’, Journal of Chemical Society of Pakistan, vol. 27, no. 3, pp. 627–630.
Carvalho, G, Delee, W, Novais, JM & Pinheiro, HM 2002,’ A factorially-designed study of physico-chemical reactive dye colour removal from simulated cotton textile processing wastewaters’, Coloration Technology, vol. 118, no. 5, pp. 215–219.
Gaehr F, Hermanutz, F & Oppermann W 1994,’ Ozonation— an important technique to comply with new German laws for textile wastewater treatment’, Water Sci.Technol., vol. 30, no. 3, pp. 255-263
Islam, MM, Mahmud, K, Faruk, O & Billah, MS 2011,’ Textile Dyeing Industries in Bangladesh for Sustainable Development’, International Journal of Environmental Science and Development. vol. 2, no. 6, pp. 428-436.
Karcher, S, Kornmuller, A & Jekel, M 2002,’ Anion exchange resins for removal of reactive dyes from textile wastewaters’, Water Research, vol. 36, no. 12, pp. 4717- 4724.
Kim, TH, Park, C, Yang, JM & Kim, S 2004,’Comparison of disperse and reactive dye removals by chemical coagulation and Fenton oxidation’, Journal of Hazardous Materials, vol. 112, no. 8, pp. 95–103.
Marmagne, O & Coste, C 1996, Color Removal From Textile Plant Effluents. Web.
Najafi, H & Movahed, HR 2009,’Improvement of COD and TOC reactive dyes in textile wastewater by coagulation chemical material’, African Journal of Biotechnology, vol. 8, no.13, pp. 3053-3059.
Ofoefule, A, Uzodinma, E & Ibeto, C 2011, Waste Water: Treatment Options and its Associated Benefits, University of Nigeria Press, Nsukka, Nigeria.
Papic, S, Koprivanac, N & Bozic, AL 2000,’Removal of reactive dyes from wastewaterusing Fe(III) coagulant’, Journal of the Society of Dyers and Colourists, vol. 116, no. 3, pp. 352–358.
Papic, S, Koprivanac, N, Bozic, AL & Metes, A 2004,’ Removal of some reactive dyes fromsynthetic wastewater by combined Al (III) coagulation/carbon adsorption process’, Dyes and Pigments, vol. 62, no. 1, pp. 291–298.
Porter, J, Deere, D, Hardman, M, Edwards, C & Pickup, RW 1997,’ Go with the flow-use of flow cytometry in environmental microbiology’, FEMS Microbial. Ecology, vol. 24, no. 2, pp. 93 – 101.
Ramesh Babu, B Parande, AK, Raghu, S & Prem Kumar, T 2007,’ Cotton Textile Processing: Waste Generation and Effluent Treatment’, The Journal of Cotton Science, vol. 11, no. 3, pp. 141–153.
Ramakrishna, KR & Viraraghavan, T 1997,’ Dye removal using low cost adsorbents’, Water Science and Technology, vol. 36, no. 2-3, pp. 189-196.
Robinson NJ, Whitehall, SK & Cavet JS 2001,’Microbial metallothioneins’, Adv Microb Physiol, vol. 44, no. 10, pp. 183-213.
Sarasa J, Roche MP, Ormad MP, Gimeno E, Puig A & Ovelleiro, JL 1998,’Treatment of a wastewater resulting from dyes manufacturing with ozone and chemical coagulation’, Water Res, vol.32, no.9, pp. 2721-1727.
Şen, S & Demirer, GN 2003,’ Anaerobic treatment of real textile wastewater with a fluidized bed reactor’, Water Res, vol. 37, no. 8, pp. 1868-78.
Szygula, A, Guibal, E, Palacin, MA, Ruiz, M & Sastre, AM 2009,’ Removal of an anionic dye (Acid Blue 92) by coagulation-flocculation using chitosan’, Journal of Environmental Management, vol. 90, no. 77, pp. 2979-2986.
Tang C & Chen V 2002,’ Nanofiltration of textile wastewater for water reuse’, Desalination, vol.143, no. 1, pp. 11-20.
Türgay, O, Ersöz, G, Atalay, S, Forss, J & Welander, U 2011,’ The treatment of azo dyes found in textile industry wastewater by anaerobic biological method and chemical oxidation’, Separation and Purification Technology, vol. 79, no. 4, pp. 26-33.
Vandevivere, PC, Bianchi, R & Verstraete, W 1998,’ Treatment and reuse of wastewater from the textile wet-processing industry: review of emerging technologies’, J. Chem. Technol. Biotechnol, vol. 72, no. 4, pp. 289–302.
Verma, AK, Dash, RR & Bhunia, P 2012,’ A review on chemical coagulation/flocculation technologies for removal of colour from textile wastewaters’, Journal of Environmental Management, vol. 93, no. 1, pp. 154-168.
Walker, GM & Weatherly, LR 1997,’ Adsorption of acid dyes on to granular activated carbon in fixed beds’, Water Research, vol. 32, no. 6, pp. 2093-2101.
Wang , Z, Xue, M, Huang, K & Liu, Z 2011, Textile Dyeing Wastewater Treatment, Huazhong University of Science and Technology Press, Huazhong.
Zahrim, AY, Tizaoui, C & Hilal, N 2010,’ Evaluation of several commercial synthetic polymers as flocculant aids for removal of highly concentrated C.I. Acid Black 210 dye’, Journal of Hazardous Materials, vol. 182, no.3, pp. 624-630.