Introduction
Osmosis is a process by which water or other fluid travels across a semi-permeable membrane from a low concentration of dissolved particles to a region of high concentration of dissolved particles. Dissolved particles can pass across a semi-permeable membrane from a region of high concentration to an area of low gathering due to differences in the concentration gradient. Pushing the fundamental understanding of osmosis allows one to propose new perspectives for different fields (Marbach & Bocquet, 2019). The aforementioned is crucial in providing significant impact in various fields.
Procedures
For three days, three eggs were placed in vinegar. Then, demineralized egg was taken out of the vinegar and cleaned with tap water. Carefully placing the cleaned egg into the spotless cup, it was weighted. The egg’s actual mass was calculated and noted. After that, distilled water and a hypotonic solution, was added to the cup to the level of three-quarters and left for 40 minutes. The water was carefully drained after 40 minutes, and the egg’s weight in the cup was calculated. The egg was then placed in a clean cup and covered with corn syrup (hypertonic solution), which was then set for 40 minutes. Once more, the solution was cautiously poured out, and the weight of the cup containing the egg was calculated and noted.
Results
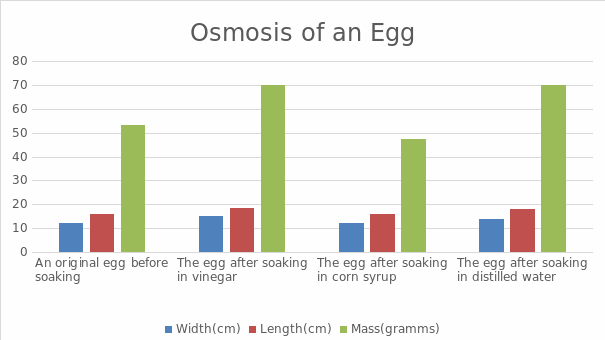
The experiment’s findings demonstrate the egg’s increased mass after being soaked in vinegar. This can be explained by the fact that vinegar contains more water than an egg, so water moved through osmosis from the vinegar’s highly concentrated area into the egg’s less concentrated area. The egg’s weight rose from 53.3g to 69.9g, as well as its length and width, which went up from 12cm to 15cm and 16cm, respectively. Meanwhile, we found that corn syrup had a lower concentration of water molecules; therefore, through osmosis, water molecules transferred from the egg into the corn syrup, causing the egg’s weight and size to drop, as shown in the table. Finally, the size and weight of the egg rise as it is submerged in distilled water. This is an evident indicator that water molecules went from a highly concentrated location through osmosis into the egg, which contained fewer water molecules.
Conclusion
Water molecules moved from a region with a high gradient to one with a lower gradient. For instance, water moved out when the egg was submerged in corn syrup, lowering the egg’s weight; with distilled water, the opposite is accurate. As a result of investigating the osmosis process, the experiment’s goal was achieved.
Reference
Marbach, S., & Bocquet, L. (2019). Osmosis, from molecular insights to large-scale applications. Chemical Society Reviews, 48(11), 3102–3144.