Reverse osmosis (desalination) is a process of purifying water using a semi-permeable membrane. In this process, a form of counter pressure is applied to overcome the osmotic pressure that is determined by chemical potential and a thermodynamic constraint. The concentrations of seawater and brackish water differ considerably; hence, there is a distinction involving the concentrate acquired from seawater desalination plants and brackish water desalination plants (Bergman, 2007).
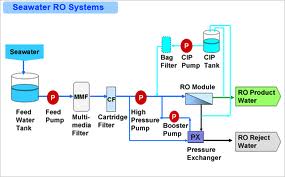
Seawater reverse osmosis systems are meant to eradicate huge quantities of salts and other mineral deposits from seawater. Seawater has a high salinity rate because it is found in areas that experience high evaporation rates mostly in the latitudes of north and south. Therefore, seawater reverse osmosis entails applying high pressure to the water that is contaminated forcing it to pass through semi-porous membranes, which prevents salts and other organics from passing. The main function of the membrane is to remove dissolved solids in the solution with an aim of separating the feed water from purified water. Seawater desalination utilizes minimal energy exploitation corresponding to the osmotic pressure multiplied by the volume of desalinated water. This energy is general and efficient to all desalination technologies that are used in water purification (Cipollina, Micale, & Rizzuti, 2007).
Brackish water contains a higher amount of salinity than fresh, but not as much as sea or ocean water. Moreover, brackish water is obtained through a mixture of fresh water and seawater, at a point where rivers run into the ocean. The brackish water reverse osmosis is a technique that removes organic wastes and impurities from water. This process is also capable of removing constituents that have a higher molecular mass. For instance, heavy metals and organic compounds are also found in brackish water, and they have a great impact on the concentration of the water. Therefore, hydraulic pressure is used to overcome a nourish solution’s osmotic pressure and induce the distribution of pure water through a semi-permeable membrane. In this case, this process is applicable in foodstuff and drink industries, potable drinking water, hospitals, farming and resorts, water bottling and ice manufacture, pharmaceuticals (Greenlee, Lawler, & Freeman, 2009).
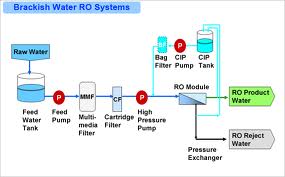
Before water starts to undergo the above treatments, there is a stage of the pretreatment process, which is aimed at reducing fouling that may cause reduction of the water flux; hence, minimizing the water recovery in the process. Pretreatment also minimizes hydrolysis and oxidation, which may result from reactions between metals and chemicals found in the feed water. Some of the processes that are involved in pretreatment include acid treatment, cartridge filtration, dechlorination, and coagulation. On the other hand, chemicals used during these processes include chlorine, sulphuric acid, sodium bisulphate, and calcium hypochlorite (Committee on Advancing Desalination Technology, 2008). In the desalination process, high concentrations of brine composed of large quantities of chemicals are discharged at the end. There are other discharges, which are obtained in the process of pretreated water that has undergone treatment, but not obtained by the required quality to pass through the membrane. The other discharge is permeating. It has not yet qualified as a final product; in fact, the permeating process has a lower salinity, and it contains very few harmful chemicals (Mauguin & Corsin, 2005).
References
Bergman, R. (2007). American Water Works Association Denver Reverse Osmosis and Naofiltration. Denver, CO: American Water Works Association.
Cipollina, A., Micale, G. & Rizzuti, L. (2007). Seawater Desalination. Conventional and Renewable Energy Processes. New York: Springer.
Committee on Advancing Desalination Technology. (2008). Desalination: A National Perspective. Washington, D.C.: The National Academies Press
Greenlee, L., Lawler, D. & Freeman, B. (2009). Reverse osmosis Desalination: Water sources, technology, and today’s challenges. Water Research. 2317-2348. Web.
Mauguin, G. & Corsin, P. (2005). Concentrate and other waste disposals from SWRO Plants: characterization and reduction of their environmental impact. Mauguin-SWRO rejects-Italy-may 05, 182(1–3), 355–364. Web.