Altered documents: introduction
Documentation is one of the factors, which indirectly contributes towards production of quality products. For an organization to manufacture quality products, precise and lucid documentation must be adhered to in the areas of manufacturing, procedure & quality system elements as well as in specification and testing. Violations or failures however occur in documentation processes, thus leading to production of products of poor quality.
Following such considerations and the importance of understanding the role played by manufacture of quality products within pharmaceutical/ medical industries, an inspection was carried out on the documentation issues for Cordis medical device manufacturing company.
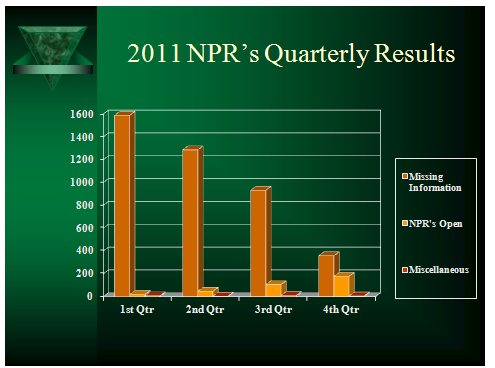
What follows below is thus a gap analysis report of the inspection results for the identified company’s documentation issues for the four quarters of the previous year, 2011; and which were categorized into three classes as missing information, open NPR’s and miscellaneous.
Missing information
During the previous year, 2011, a total of 1589, 1286, 932 and 358 missing information issues were reported for the four quarters of the year respectively. In other words, these quarterly figures represented 38.15%, 30.86%, 22.38% and 8.60% of the total 4165 annual figure, and having average quarterly reported issues of 1041.
This vividly depicts that there is a wide range on the number of issues reported quarterly, whereby the highest number of issues is reported during the first quarter, while the least figure is obtained at last quarter of the year.
The annual figure of 4165 of missing information issues were as contribution from area of identification, investigations and disposition matters. The difference observed in this may be contributed perhaps by several factors including personal ones like education and experience, work environment (volumes of work) among others.
Miscellaneous
For the Miscellaneous cases, it was found that there were 7, 4, 10 and 5 reported issues for the four quarters of year in ascending order. With the sum of 26 issues in this category, 18 of these were reported as overwrites while the rest of the remaining issues were either cross-outs or not assigned matters of NPR’s.
Generally, it appears that the resulting differences of each of the individual reported overwrite case for the various quarters are not quite significant.
However, violations of overwrite type were at the peak during the third quarter, but minimally experienced problem between the months comprising the second quarter of the year. This is indication on review and audits records such as production batch records and procedures of processes may be affected variably during certain time of the year
Open NPRs
Focusing on the issue of open NPR’s, quarterly deficiencies were reported as 19, 47, 105 and 177. The open NPR’s problem figures increase from the first quarter to the last one, hence giving an increasing track and trend of the problem.
Impact: what impact does the variable have on the product?
Following the results obtained from the investigative study of the three variable levels contributing towards poor documentation within the Cordis organization, we have found that much of the essential information need for manufacture and improve the quality of the organization’s products is not made available as required to right parties.
On the overall focus of the variables that were investigated for the Cordis company, one identifies that all of the three variables would affect the quality of the company’s products in one way or another.
This mainly includes the information contained or describing the product’s originality, processes and procedures followed in their manufacture which are necessary for authorization for human use, and which affect their legal authorization in marketing distribution in certain regions.