History of Gene Therapy
The field of research concerning the modification of cells to cure certain diseases became known in the early 1970s. Robin and Friedmann were the first to publish an article concerning gene therapy (Friedman, 1992). The research by Robin and Friedmann cited Rogers’ early works in 1970 that proposed the utilization of exogenous DNA to reconstruct non-functioning DNA. In the 1980s, scientists developed a retrovirus vector structure, and it was effective in penetrating and depositing foreign DNA materials that were compatible with other mammalian cells (“Gene therapy basics,” 2021). In the 1990s, the United States approved its first gene therapy (Wirth et al., 2013). The first patient was a four-year-old suffering from an acute immune system deficiency (adenosine deaminase deficiency (ADA)). Treatment was successful; however, the effects were temporary, and the child had to return for therapy after every two months. In 1992, gene therapy was performed to cure ADA in children (“Gene therapy,” 2021). The success of the procedure was then published and performed in 2002. However, there were minor complications that led to its stoppage. Due to technological advancements, scientists continued with this procedure within the same year.
- Gene therapy began in the 1970s with its publication concerning its potential capabilities
- In the 1980s, there was the development of a retroviral vector system that could deposit new genetic materials into a defective cell
- The United States approved the first gene therapy in the 1990s, which led to the successful but temporary treatment of ADA
- In the early 2000s, the field of gene therapy saw development with numerous successes in treating ADA; however, some complications led to its stoppage. With technological advancements, gene therapy continued with cancer being one of the conditions that could be treated
- In 2002, researchers conducted a study involving the treatment of sickle-cell
- 2003 was the year that scientists decided to find out whether gene therapy could be used to treat neurological disorders
- Scientists discovered that the procedure could treat the myeloid in 2006
- From 2007 to 2021, research has shown that numerous conditions could be treated using the procedure, including the use of an altered version of HIV in treating children with ADA-SCID (“Gene therapy,” 2017)
Description of Gene Therapy
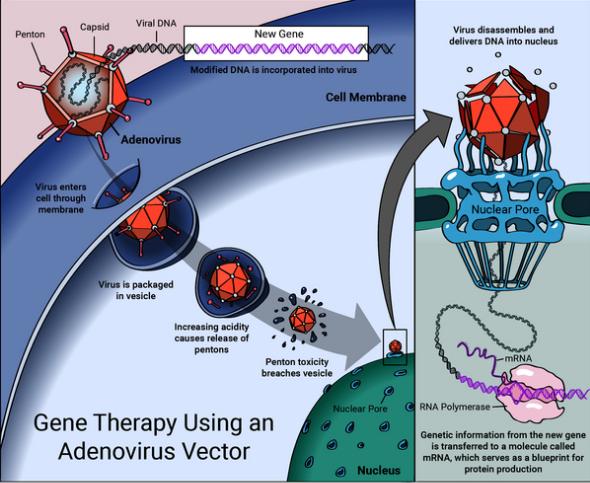
Gene therapy refers to a field of medicine that primarily focuses on genetically modifying cells to generate an effect that is therapeutically in nature. In other words, Cross (1999) describes it as a type of treatment that involves reconstructing or modifying defective gene materials. Furthermore, it can be described as a type of treatment that is experimental and utilizes genetic material gene transfer into a sick person to cure certain illnesses. The theory, in this case, is to reconstruct the genetic data of the cell of the sick person that is causing the disease. The goal of this modification is to attempt to restore the normal functionality of that particular cell. To return a cell’s normal functionality, physicians use viral vectors (“Gene therapy basics,” 2021). Viral vectors can penetrate human cells and deposit the required genetic materials. Gene therapy is important because it provides solutions for treating acquired and inherited disorders.
Steps of Gene Therapy
- Obtaining genetic materials
- The next step is to insert a new gene directly into a particular cell
- The inserted adenovirus then reconstructs the defective cell by introducing DNA within the cell’s nucleus; however, the DNA does not integrate with the chromosome.
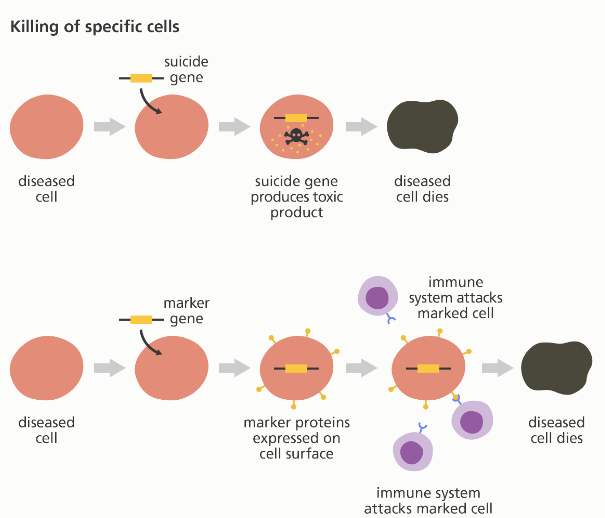
Products of Gene Therapy that Treats Cancer
The area of gene therapy has been facing challenges when it comes to defining a path to the market and the clinic. According to Mandal (2019), over 1500 clinical trials have been performed, and roughly 20 approved gene therapy products are currently available. The advantage of this is that currently, there are numerous opportunities in treating incurable and inherited conditions (Verma et al., 2000). Today gene therapy has become profitable, with most of its products being available on the market. Furthermore, there has been an increase in the funding of clinical trials, which makes it possible for scientists to explore further possibilities of treating such diseases as cancer and ADA. One of the products that have been approved for usage in the market is Vitravene. The product is utilized to treat CMV retinitis, which is a condition that affects the eye, particularly in patients suffering from AIDs. Research states that the product can successfully eliminate the symptoms of CMV retinitis, thereby helping patients cope with such conditions.
The other important product in the market is Gendicine, which treats head and neck squamous cell carcinoma (HNSCC). The drug is essential because it contains properties that stimulate the production of antitumor materials. Gendicine was tested and approved in 2003 and is effective in preventing different tumors from forming. Treatment using this product was found to be effective and improved patients’ health. Their overall survival rates from head and neck cancer were better compared to individuals that did not use the product (McCain, 2005). The product is available in the worldwide market and is currently being utilized by different individuals. The other product is Oncorine which is utilized to treat colon, breast, and head cancer. According to research, there are types of cancer that do not respond to chemotherapy (Mendell et al., 2021). In this case, Oncorine is capable of initiating an immune response that would increase the chances of responding to chemotherapy. As a result, it improves the survival of patients suffering from lung and neck cancer.
Risks of Gene Therapy
Despite the many advantages, there are several risks associated with gene therapy. The complications of gene therapy are mainly related to the way they are inserted into the human body. The procedure involves utilizing a carrier to transport new genetic materials into the cell. Physicians usually use viruses as vectors since they can identify certain cells within the human body (NIH, 2020). In this case, scientists remove the section that causes disease within the virus and replace it with a new one intended to cure the person’s condition (“What is gene therapy?: MedlinePlus genetics,” 2021). One of the most common side effects of gene therapy is an unwanted response to the newly introduced virus. In some situations, the body may treat these new materials as unwanted viruses and, as a result, the body defense mechanism attacks them. This reaction may lead to severe organ failure and inflammation (Shahryari et al., 2019). The other risk is that the carriers may target other cells. Viruses can invade different cells which may cause additional complications. The altered cell may affect healthy ones in the body, thereby causing damage that may lead to the development of cancer.
The use of a disease-causing virus is further a risky approach to gene therapy. Even though scientists remove the disease-causing part of the virus, some carriers can regenerate after being introduced into the body. As a result, they regain their original capacity to cause the disease, thereby affecting the patient. In addition, there are some situations where the new genetic materials may be inserted into the wrong cell (U.S. Food and Drug Administration, 2018). When this occurs, there are chances that the patient may develop a tumor. therefore, despite having numerous advantages such as increasing chances of survival, there are situations where a patient can develop other serious conditions. Nevertheless, these are rare instances primarily because there have been numerous technological advancements within the gene therapy fields.
The Future of Gene Therapy
While there are several advancements in the gene therapy field, numerous enhancements should be considered. According to the U.S. Food and Drug Administration (2017), current gene therapy procedures require autologous cells to produce vaccines. These autologous cells are an effective method of providing care to individuals with serious conditions. However, they are very expensive to manufacture, thereby making it costly for patients and researchers to purchase and produce. Furthermore, very few hospitals have enough facilities and resources to create such vaccines. In addition, their product requires a substantial amount of expertise to produce (“Gene therapy’s promise: Future uses, applications & prospects | Pfizer,” 2021). Therefore, in the future, scientists must utilize allogeneic vaccines to treat inherited diseases. Allogeneic vaccines do not have the same effects as autologous vaccines when it comes to treating some conditions; however, they have been proven to be effective in treating some diseases that produce autologous tumors. In addition, treatment of cancer should combine another form of therapy to ensure that cancer cells are eliminated from the body. Currently, there is the development of vaccines against acquired conditions.
The future of gene therapy is promising with researchers currently exploring the different ways of improving viral vectors. According to Cross (1999), viruses are capable of targeting different types of cells including healthy ones. To ensure that there are reduced chances of this taking place, it is important to ensure that gene modification is improved. Clinical trials are currently studying ways in which carrier cells can be improved to successfully deliver the new genetic materials without regenerating them. This development is important because it would ensure that there are no additional conditions that may arise from gene transfer (“What is gene therapy?,” 2017). In addition to this, due to technological advancements, scientists have been able to develop new products that help in treating server conditions. Governments, especially those of developed countries have increased funding towards the discovery of more vaccines to help manage such diseases as lung and neck cancer.
Gene therapy is a form of medication that allows one to manage severe diseases. The treatment method involves altering viruses by removing the section that causes disease and replacing it with genes meant to cure a particular condition. The procedure began with a publication concerning how to treat severe conditions through altering DNA. Since the 1970s, gene therapy has undergone changes that have been beneficial to human beings. Patients have been able to manage the condition with ease and their chances of survival have further increased. Despite these improvements, there have been some challenges associated with the process. The use of viruses may cause other healthy cells to be affected, thereby causing cancer or inflammation. There are possibilities of targeting the wrong cells, which may further cause complications within the body.
References
Cross, D., & Burmester, J. K. (2006). Gene therapy for cancer treatment: Past, present and future. PubMed Central (PMC). Web.
Cross, M. (1999). Human gene therapy. NDSU – North Dakota State University. Web.
Friedman, T. (1992). A brief history of gene therapy. Nature. Web.
Gene therapy basics. (2021). Gene & Cell Therapy Education | ASGCT – American Society of Gene & Cell Therapy |. Web.
Gene therapy. (2021). WhatisBiotechnology.org. Web.
Gene therapy. (2017). Mayo Clinic – Mayo Clinic. Web.
Gene therapy’s promise: Future uses, applications & prospects | Pfizer. (2021). Pfizer: One of the world’s premier biopharmaceutical companies. Web.
Mandal, A. (2019). Gene therapy history. News-Medical.net. Web.
McCain, J. (2005). The future of gene therapy. PubMed Central (PMC). Web.
NIH. (2020). Gene therapy. Genome.gov. Web.
Shahryari, A., Jazi, M. S., Mohammadi, s., Nikoo, H. R., Nazari, Z., Hosseini, E. S., Burtscher, I., Mowla, S. J., & Lickert, H. (2019). Development and clinical translation of approved gene therapy products for genetic disorders. Frontiers. Web.
U.S. Food and Drug Administration. (2017). What is gene therapy? How does it work? Web.
U.S. Food and Drug Administration. (2018). FDA approves novel gene therapy to treat patients with a rare form of inherited vision loss. Web.
Verma, I. M., Naldini, L., Kafri, T., Miyoshi, H., Takahashi, M., Blömer, U., Somia, N., Wang, L., & Gage, F. H. (2000). Gene therapy: Promises, problems and prospects. Genes and Resistance to Disease, 147-157. Web.
What is gene therapy? (2017). yourgenome.org | Helping you discover more about DNA, genes and genomes, and the implications for our health and society. Web.
What is gene therapy?: MedlinePlus genetics. (2021). MedlinePlus – Health Information from the National Library of Medicine. Web.
Wirth, T., Parker, N., & Ylä-Herttuala, S. (2013). History of gene therapy. ScienceDirect.com | Science, health and medical journals, full text articles and books. Web.