DNA profiling at birth is essential toward supporting crime investigations that may occur in the future. DNA profiling involve analyzing short tandem repeats (STRs) on chromosomes that are determining factors of identity, and useful toward providing supportive evidence in forensic investigations. It is essential to conduct genetic tests at birth, and store the information in databases to support possible crime investigation activities in the future. Genetic testing in the United States is facing challenges unlike in Great Britain (Aronson, 2007). Ethical issues prohibit conduction of research on children without the consent of the parents (Nelson et al., 2001).
DNA profiling at birth will solve many cases in the courtroom through easy identify of criminals, and placement of a justified verdict in reference to the people v. Castro law case (Hoeffel, 1990). The government should make an informed research before making a final decision on expanding the DNA database for all citizens because of possible hackers, and ethical issues regarding the storage of DNA profiles (Williams & Johnson, 2005).
Issues
Nelson et al. (2001) states that, DNA profiling of children is under consideration due to lack of general acceptance by the scientific community, especially in violation of human rights. The multi-stage process of genetic testing is time consuming, requires expertise, and may create an element of doubt on the credibility of the results due to DNA degradation. One of the DNA profiling methods is called restriction fragment length polymorphism (RFLP) as shown in Figure 1.
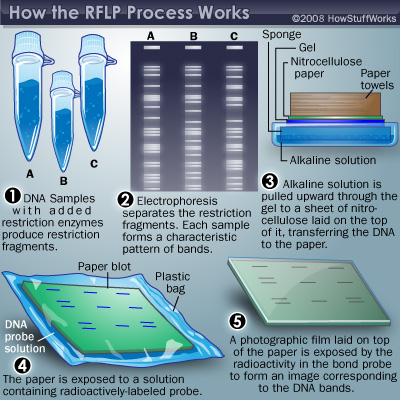
RFLP is a multi-step process, and each step creates a chance of human error leading in lack of reliability of results for court evidence. Other methods used for genetic testing are polymerase chain reaction, and amplified fragment length polymorphism (Daves, 1991; Alonso et al., 2005). The courts should not only rely on scientific evidence in making final decisions because science blindfolds the society. According to Hoeffel (1990), judges should incorporate scientific evidence, and conventional ways toward making a final decision.
The genetic testing requires caution since coincidental match may occur in one-in-a ten thousand chances according to the odds of a random occurrence for each band. Additionally, forensic investigations require the use of approved laboratory testing procedures in order to use the evidence in the court (Williams & Johnson, 2005; Aronson, 2007).
Recommendations
The United States government has a role of validating the DNA profiling laboratory procedures, and giving consent to the children at birth to undergo genetic testing. The government should also generate a theory that is accepted by the scientific community and supports conclusions of the DNA profiling.
The management of DNA profiles is a challenge to the government bodies. Databases should be secured in order to protect the genetic information. Alonso et al. (2005) explain an improved technology that allows the application of Bioinformatics tools in making pairwise comparisons of DNA profiles as shown in figure 2.
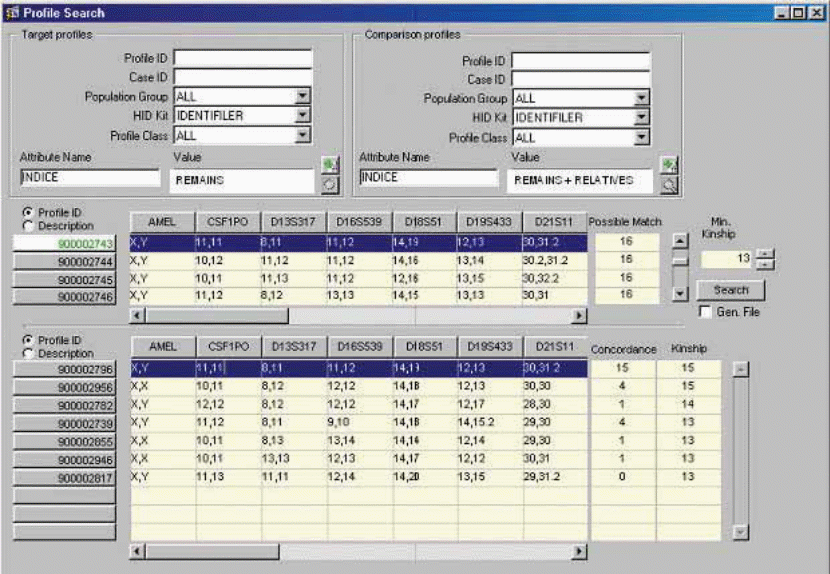
The reliable tests for conducting genetic testing should be more than one in order to remove the element of doubt on matching DNA bands. All genetic testing for crime investigations should have approved controls for the purpose of producing reliable results (Daves, 1991; Alonso et al., 2005).
Reasoning
Williams & Johnson (2005) argue that, DNA profiling tests are reliable for court evidence if there is a partial match of at least ten loci out of thirteen loci. A single genetic testing at birth may not provide all the possible crime investigation activities in the future due to chances of DNA mutation, and degradation. Additionally, genetic tests are expensive to impose on every citizen. DNA profiling results require a deep inspection, and data interpretation to create reliability in crime evidence.
The advanced technique of conducting DNA profile is the use of molecular probes synthesized from bacteria. The molecular probe technique lacks contaminants of non-human origin; this results in the elimination of ambiguities in analysis of the results (Hoeffel, 1990; Daves, 1991).
The probes present in DNA profiling should be familiar to the experts providing the evidence in the law courts. Daves (1991) challenges the government toward standardizing, and clarifying the use of DNA profiling as novel scientific evidence in the courts. In some situation, the stored analysis of DNA profile in the database from newborns may not provide acceptable measurements of precision.
The government has the mandate of stating precisely the characteristics identified in the suspect, and perpetrator sample (Williams & Johnson, 2005). Describing the characteristics of the genetic profile results such as, the existence of DNA bands, the band size, intensity of the bands, and restriction enzyme is essential in court evidence (Daves, 1991; Alonso et al., 2005).
The reasons for these decisions are that DNA profiling characteristics cannot change unpredictably. External conditions do not have a legitimate basis for the genetic testing results because of possible errors as shown in figure 3. External factors such as temperature affect the DNA yield and characteristics, hence resulting in varying results (Aronson, 2007). High temperatures degrade DNA and results to low yield.
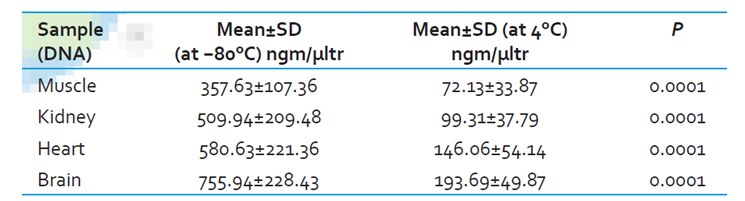
It is unfortunate that samples collected at the crime scene undergo significant changes, hence giving varying results with the stored DNA profiles of newborns (Daves, 1991; Nelson et al., 2001). Scientists apply precise, and accurate measurements in diagnosing genetic diseases, especially in newborns (Nelson et al., 2001). Similar precision should be applied in forensic research. Depending on the evidence present at the crime scene, the test results should match the stored DNA profile.
Presence of dissimilar match between two samples creates an evidence that the culprit, and the perpetrator are different, hence the need of handling the samples at crime with great precaution (Williams & Johnson, 2005; Aronson, 2007).
Implications
Williams and Johnson (2005) argue that, results of identical match after genetic testing must have the supportive rational inference that explains that the match could not have occurred in a random. Calculations are essential toward determining the probability of occurrence of coincidental results in the suspect population.
The information on the probability of occurrence helps in determining the significance of the match (Aronson, 2007). For example, a characteristic present on every individual cannot be used to declare the match because it lacks probative value. Government intervention on identification of evidence from the scientific perspective is helpful in calculating the odds of a coincidental match.
For example, a hair sample at crime scene can easily indicate whether the perpetrator was a male or female, hence giving a percentage of fifty in the population (Daves, 1991). Misinterpretations of the genetic testing results lead in the creation of doubt, and reliability of the tests as the sources of evidence in crime investigation activities.
However, new technologies in scientific investigations have a high probability of producing reliable DNA profiling results that are useful for forensic investigations. According to Nelson et al. (2001), possible side effects of DNA profiling of newborns, and storing in the database are hacking on the DNA database. Cases of framing for murder or criminal acts are likely to increase in situations where hackers break through the government DNA databases (Daves, 1991).
Some forensic experts suggest storing of DNA profiles of convicts, perpetrators, and suspects, but not innocent people. The government may face the challenge of ethical consideration, and violation of personal privacy from the human activist societies. DNA profiling of newborns is like giving the government the authority in using the genetic profile without individual consent (Nelson et al., 2001; Aronson, 2007).
The community reaction is a lack of acceptance of each element present in the genetic profiling test procedure. Nelson et al. (2001) challenge the government to intervene on the response of the community, and formulate laws that create credibility on the DNA profiling procedures of newborns that will result in the expansion of DNA databases.
The government has a mandate of increasing DNA database, and establish safety to avoid data manipulation. DNA profiling of newborns will solve many criminal cases, and increase the health status of the American citizens. Criminals will have little chance of hiding, and the police will have ease in identifying the culprits (Williams & Johnson, 2005; Alonso et al., 2005).
References
Alonso, A., Martín, P., Albarrán, C., de Simon, P. L. F., Iturralde, M. J., Fernandez-Rodriguez, A., Atienza, I., Capilla, J., Garcia-Hirschfeld, J. & Martinez, P. (2005). Challenges of DNA profiling in mass disaster investigations. Croatian medical journal, 46(4), 540-548.
Aronson, J. (2007). Genetic witness: science, law, and controversy in the making of DNA profiling. Piscataway, NJ: Rutgers University Press.
Daves, A. (1991). The use of DNA profiling and behavioural science in the investigation of sexual offences. Medicine, Science and the Law, 31(2), 95-101.
Hoeffel, J. C. (1990). The Dark Side of DNA Profiling: Unreliable Scientific Evidence Meets the Criminal Defendant. Stanford Law Review, 42, 465-538.
Nelson, R. M., Botkin, J. R., Kodish, E. D., Levetown, M., Truman, J. T., Wilfond, B. S. & Steinberg, D. (2001). Ethical issues with genetic testing in pediatrics. Pediatrics, 107(6), 1451-1455.
Williams, R., & Johnson, P. (2005). Inclusiveness, effectiveness and intrusiveness: issues in the developing uses of DNA profiling in support of criminal investigations. The Journal of Law, Medicine & Ethics, 33(3), 545-558.